
CiPA Screening
Evaluation of the proarrhythmic liability of new drug candidates
Introduction to CiPA
The International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs. They were implemented in response to the discovery that inhibition of the rapid delayed rectifier potassium current (IKr), which is encoded by the human ether-à-go-go related gene (KV11.1), is associated with prolongation of the QT interval and the potentially deadly arrhythmia, Torsades de Pointes.
The guidelines utilise hERG inhibition and QT interval prolongation as surrogate markers of proarrhythmic liability, which are highly sensitive and have proven effective at preventing proarrhythmic drugs from reaching the market. However, these markers have low specificity, with only a modest correlation between hERG inhibition, QT prolongation and proarrhythmic liability. Therefore, to address these limitations, the Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative was launched by the Food and Drug Administration (FDA) in July 2013. The CiPA initiative aims to improve the accuracy and reduce the cost of predicting cardiac liability using three ‘pillars’:
- Compounds will be profiled against a panel of human ventricular ion channels.
- This in vitro data will be incorporated into an in silico model of a human action potential to provide a proarrhythmic risk classification.
- Compounds will be tested using human induced pluripotent stem cell- derived cardiomyocytes to confirm the risk classification derived from the in silico model.
We have developed a comprehensive panel of CiPA compliant assays and additional assays that provide an excellent in vitro evaluation of cardiac risk.
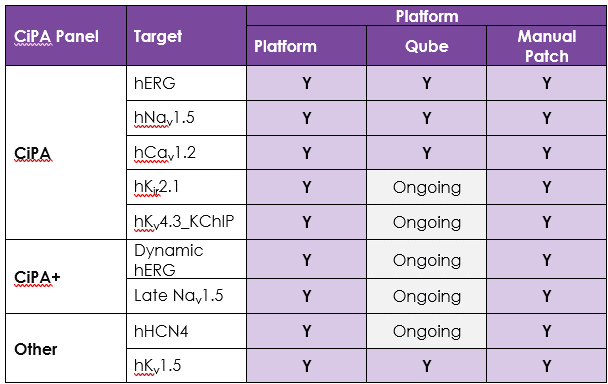
Comprehensive ion channel panel
An active participant in the CiPA ion channel HTS sub-team, we are also a member of the Health and Environmental Sciences Institute (HESI) cardiac committee. We worked closely with HESI to help reduce data variability between screening sites and to provide validation data to support efforts to generate predictive in silico models. Through these activities, Metrion has validated assays for a wide range of different human ventricular ion channels, which includes the full CiPA panel: hERG, peak and late Nav1.5, Cav1.2, KCNQ1/KCNE1, Kir2.1 and Kv4.3 (Table 1). We have also developed a dynamic hERG assay, the data from which can be used to improve the accuracy of the FDA model.
Screening services against HCN4 and Kv1.5, which play important roles in controlling human heart rate and atrial repolarisation, respectively, are also provided.
Table 1. Assays for a wide range of different human ventricular ion channels.
Potency assessments against each cardiac ion channel
We provide potency assessments against each cardiac ion channel using single-point or four-point concentration-response assays using:
- The QPatch48 automated patch-clamp platform.
- Gold standard manual patch-clamp methodology.
The potency data derived from these high-fidelity platforms are suitable for use in in silico action potential models, which is the second ‘pillar’ of the CiPA initiative.
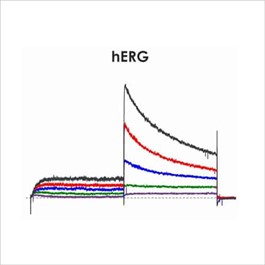
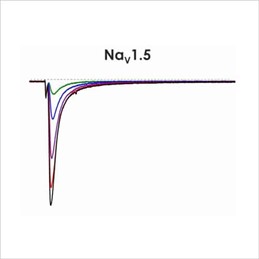
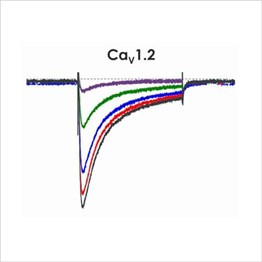
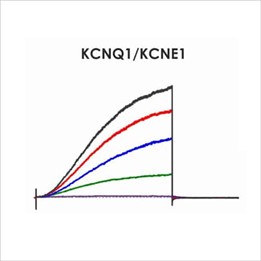
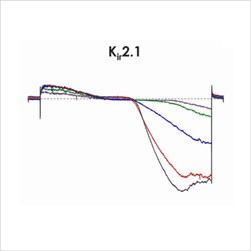
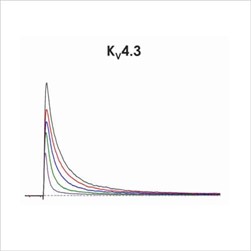
Assays for hERG, NaV1.5 and CaV1.2
Cardiac screening assays have been developed against hERG, NaV1.5 and CaV1.2 on the Qube, a 384 well automated patch-clamp platform. A number of different assay configurations are available.
Dynamic hERG assay
New screening assays can be created from existing cell lines. As part of our efforts to create an expanded panel of CiPA-compliant cardiac safety screening assays, we have developed and validated an optimised version of the very challenging ‘Milnes’ dynamic hERG voltage protocol suitable for automated patch clamp.
Translational cardiac safety assays
Clinical QTc/QRS prediction using hiPSC derived cardiomyocytes
QTc and QRS liabilities are a serious concern when developing novel clinical compounds. Assessment of ion channel activity provides a robust method to highlight potential risks, however it may not be sufficient to capture all potential mechanisms that could induce QTc, QRS or arrhythmia issues. In such cases an integrated system such as the hiPSC cardiomyocyte (hiPSC-CM) model can be a valuable model.
We can assess compounds for such liabilities in hiPSC-CMs in a higher throughput 96-well plate-based format. Using a voltage sensitive fluorescent dye, we can simultaneously measure action potential waveforms with high fidelity across all wells using the Lumencor Volta high frequency (10kHz) plate reader. This allows us to accurately capture endpoints such as action potential duration (e.g. APD90), rise time and beat rate. Moreover, this system allows for the assessment of compounds over extended time periods (up to 72h) in serum free conditions.
A key aspect of this model is its ability predict a compounds propensity to generate a prolongation in the clinical QTc interval. Moreover, that assay can help predict the free clinical exposure of a novel compound that would be associated with a 10 ms change in clinical QTc. Similarly, this model can also define the probability of a QRS clinical liability.
Further reading: Characterization of a high throughput human stem cell cardiomyocyte assay to predict drug-induced changes in clinical electrocardiogram parameters, European Journal of Pharmacology, Volume 912, 2021.
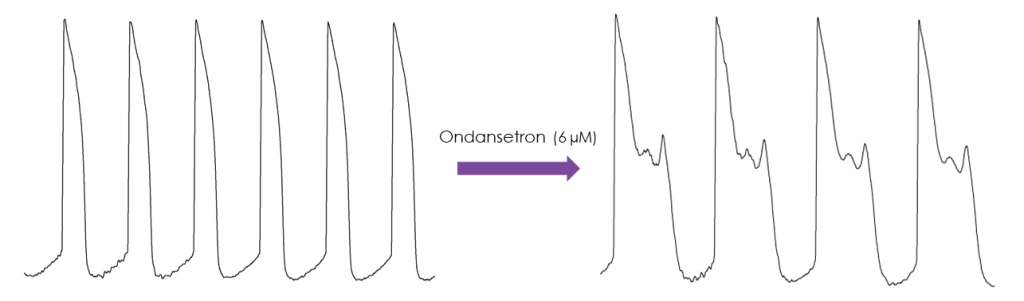
iPSC-derived cardiomyocyte screening using conventional manual patch-clamp
Services to evaluate the effect of compounds on action potentials recorded from iPSC-derived cardiomyocytes using conventional manual patch clamp methodology are also provided. Spontaneous or evoked action potentials can be recorded and used to determine the effect of compounds on a range of action potential parameters. The recordings are stable for >30 minutes, which allows the cumulative application of multiple concentrations of each compound.
The manual patch clamp assay generates high fidelity recordings that allow the detection of even subtle changes to the action potential waveform. This helps to successfully discern between compounds with low, medium and high proarrhythmic risk profiles. For example, the figures below show mean data generated using 50 nM dofetilide, which reveals a significant prolongation of all measured APD values.
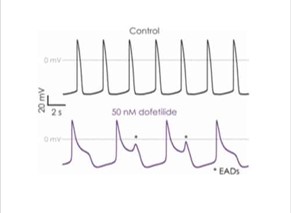
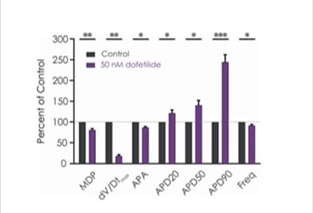
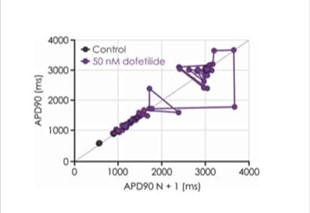
Figure 3a. iPSC-derived cardiomyocyte action potential screening.
Figure 3b. Drug effects on iPSC-derived cardiomyocyte responses.
Figure 3c. Changes in iPSC-derived cardiomyocyte beat stability.
Chronic cardiotoxicity assay – hiPSC derived cardiomyocytes
Base impedance, an indicator of cell viability, can be used to non-invasively identify structural and functional cardiotoxicity over a chronic time course. We have developed a chronic cardiotoxicity assay using human iPSC-derived cardiomyocytes, which has been validated with a number of cardiotoxicants. For example, doxorubicin, a member of the anthracycline family that is used to treat breast cancer, is associated with a number of cardiac side effects, which includes acute atrial and ventricular arrhythmias, chronic cardiomyopathy and congestive heart failure. Our chronic cardiotoxicity assay recapitulates doxorubicin’s cardiotoxic effect by producing a concentration-dependent decrease of base impedance that develops following a 24 hour exposure period.
This protocol is designed to measure the potential for test compounds to become trapped inside the hERG channel pore, and it is the first to be validated on an automated patch clamp platform.
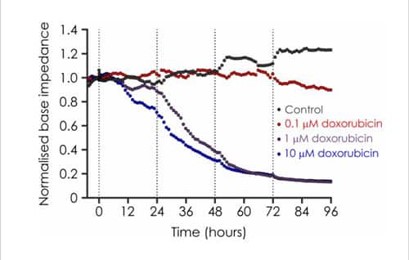
Figure 4. Effects of Doxorubicin on iPSC impedance.
Technologies used for CiPA screening
- Qube
- QPatch 48
- Gold standard manual patch-clamp
Cardiac Safety Screening Resource Library
White Papers
Application notes
- CiPA hERG Milnes Kinetic Assay on Qpatch
- Nav1.5(late) Cardiac Safety Assay on Qpatch
- Nav1.5-ΔKPQ late INa current properties and pharmacology on the SyncroPatch 384i
Publications
- A systematic strategy for estimating hERG block potency and its implications in a new cardiac safety paradigm.
- Cross-site and cross-platform variability of automated patch clamp assessments of drug effects on human cardiac currents in recombinant cells.
Posters
- The Nav1.5 Late Current in WT and Nav1.5 ΔKPQ Mutant Channels: An Automated Patch Clamp LQT3 Electrophysiological Assay Comparison. Safety Pharmacology Society Virtual Meeting 2020.
- New CiPA Cardiac Ion Channel Cell Lines and Assays for In Vitro Proarrhythmia Risk Assessment. Japanese Safety Pharmacology Society Meeting, Tokyo, 2020
- Using new In Vitro Cardiac Ion Channel Assays and In Silico Models to Predict Proarrhythmia Risk with Automated Patch Clamp. Biophysical Society Annual Meeting, San Diego, 2020
- Development of an Impedance Based Screening Assay for Cardiac Safety and Cardiotoxicity Detection in Stem Cell derived Cardiomyocytes. Safety Pharmacology Society Annual Meeting, Barcelona, 2019
- Predicting Cardiac Proarrhythmic Risk Exclusively Using Automated Patch Clamp Data. Safety Pharmacology Society Annual Meeting, Barcelona, 2019
- New CiPA cardiac ion channel cell lines and assays for in vitro proarrhythmia risk assessment. Safety Pharmacology Society meeting, Washington DC, USA 2018.
- Assessment of human induced pluripotent stem cell-derived cardiomyocytes for evaluating drug-induced arrhythmias with multi-electrode array. Safety Pharmacology Society meeting, Washington DC, USA 2018.
- Refining in vitro QPatch cardiac ion channel QPatch and MEA iPSC cardiomyocyte assays for CiPA. SOT San Antonio 2018 poster Late Breaking 12: Safety Assessment: Pharmaceutical and Non-Pharmaceutical.
- CiPA update: Refining in vitro cardiac ion channel assays, in silico models and iPSC cardiomyocyte reagents for improved proarrhythmia risk prediction. SPS Vancouver September 2016 poster 0100.
- Human ventricular stem cell cardiomyocytes: validating in vitro assays and screening platforms for proarrhythmia risk prediction. SPS Vancouver September 2016 poster 0242.
- Human stem cell-derived cardiomyocytes: in vitro assays and screening platforms for exploring ventricular and atrial phenotypes. SPS Vancouver September 2016 poster 0247.
- Electrophysiological profiling of Axiogenesis CorV.4U iPSC-derived cardiomyocytes. Axiogenesis Workshop Cologne September 2016.
- Developing a package of in vitro human cardiac ion channel assays using automated patch clamp to predict clinical arrhythmia risk. SPS Prague September 2015.
Flyers
Videos
- Presentation by Marc Rogers (Metrion CSO) at the June 2016 Sophion Ion Channel Modulation Symposium, Clare College, Cambridge (UK). CiPA update: in vitro cardiac ion channels screens, in silico models and stem cells iPS cardiomyocyte assays for pro-arrhythmia risk prediction.
- Presentation by Marc Rogers (Metrion CSO) at the September 2015 Safety Pharmacological Society, Prague. Using high quality HTS automated patch clamp data from human cardiac ion channels and in silico action potential modelling to cost-effectively predict QP prolongation and arrhythmia risk for CiPA.
Cardiac Safety Screening Technologies
- QPatch automated electrophysiology platform
- Patchliner automated electrophysiology
- Conventional manual patch clamp electrophysiology
- Plate-based impedance and microelectrode array techniques
- FlexStation plate-based imaging

Let’s work together
What are your specific ion channel screening requirements?
If you have any questions, or would like to discuss your project, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.

