The Nav1.5 Late Current in WT and Nav1.5-ΔKPQ Mutant Channels: An Automated Patch Clamp LQT3 Electrophysiological Assay Comparison
Case Study
Case Study title
The Nav1.5 Late Current in WT and Nav1.5-ΔKPQ Mutant Channels: An Automated Patch Clamp LQT3 Electrophysiological Assay Comparison
Authors
Marc Rogers¹, Maria G. Rotordam², Markus Rapedius², Robert Kirby¹, Ayesha Jinat¹, Alison Obergrussberger², Nadine Becker², Michael George², and Niels Fertig²
- Metrion Biosciences Ltd, Riverside 3; Suite 1, Granta Park, Cambridge, CB21 6AD, United Kingdom
- Nanion Technologies, Munich, Germany.
Introduction
The cardiac late Na+ current (late INa) generates persistent inward currents throughout the plateau phase of the ventricular action potential and is an important determinant of repolarisation rate, EADs and arrythmia risk¹. As inhibition of late INa can offset drug effects on hERG and other repolarising K⁺conductances, it is one of the key cardiac channels in the Comprehensive in vitro Pro-arrythmia Assay (CiPA) panel being developed by the FDA to improve human clinical arrythmia risk assessment²̛ ³.
The standard CiPA late INa assay uses the anemone toxin ATX-II to pharmacologically inhibit inactivation and produce persistent openings of wildtype (WT) Nav1.5 channels, but this method is variable and non-physiological. In contrast, several mutations in the SCN5A gene cause a form of hereditary long QT syndrome (LQT3) by promoting late openings⁴. The ΔKPQ mutation deletes residues Lys 1505, Pro 1506 and Gln 1507 and results in a sustained, non-inactivating current during long depolarizations which causes prolongation of the action potential and can result in fatal ventricular arrhythmias such as Torsade de Pointes (TdP)⁵̛ ⁶. Here we utilised a stable Nav1.5 LQT3 mutant cell line to optimise and validate a high throughput automated patch clamp late INa assay on the SyncroPatch 384i platform. High quality gigaseal recordings were obtained with a high success rate, enabling the efficient and accurate determination of relevant biophysical and pharmacological properties of this CiPA-compliant late Nav1.5 assay. The combination of the SyncroPatch 384i automated patch clamp system and Nav1.5 ΔKPQ cell line created a reliable high throughput cardiac safety screening assay without the need for openers like ATX-II toxin.
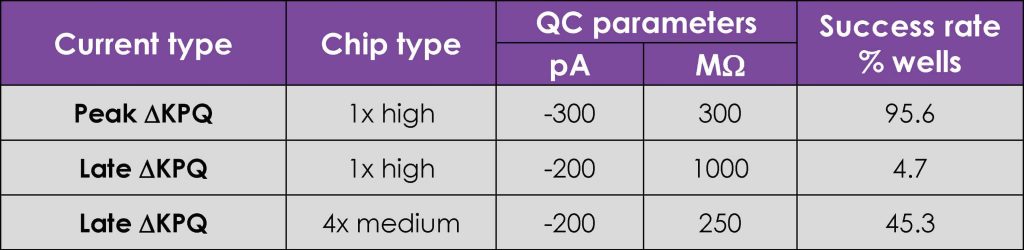
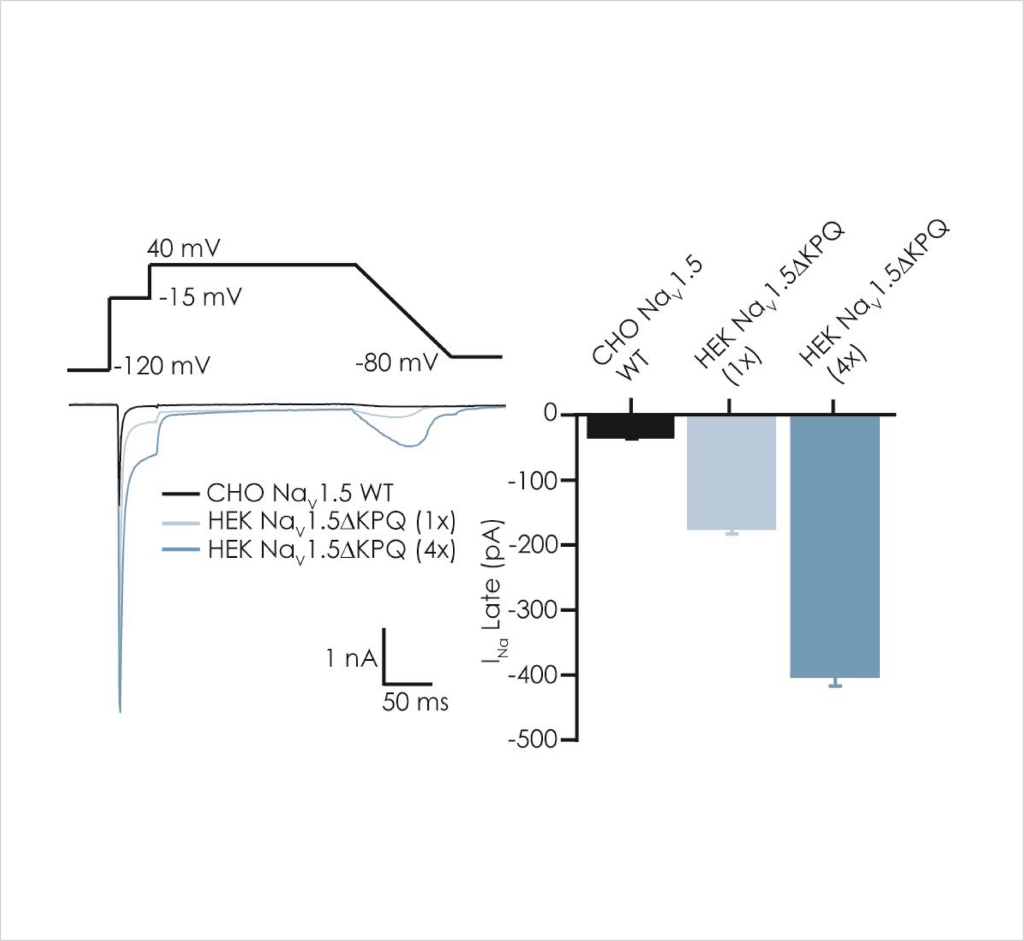
Very little optimisation of standard Nav1.5 cell line preparation and SyncroPatch384i APC assay conditions were required to achieve acceptable success rates for peak INa recordings, as measured by sealing and patchability QC parameters and current expression levels (Fig. 1). In contrast, resolving inward late currents in the ΔKPQ cell line was more challenging but a ~50% QC success rate was achieved using 4x multi-hole chips (Table 1).
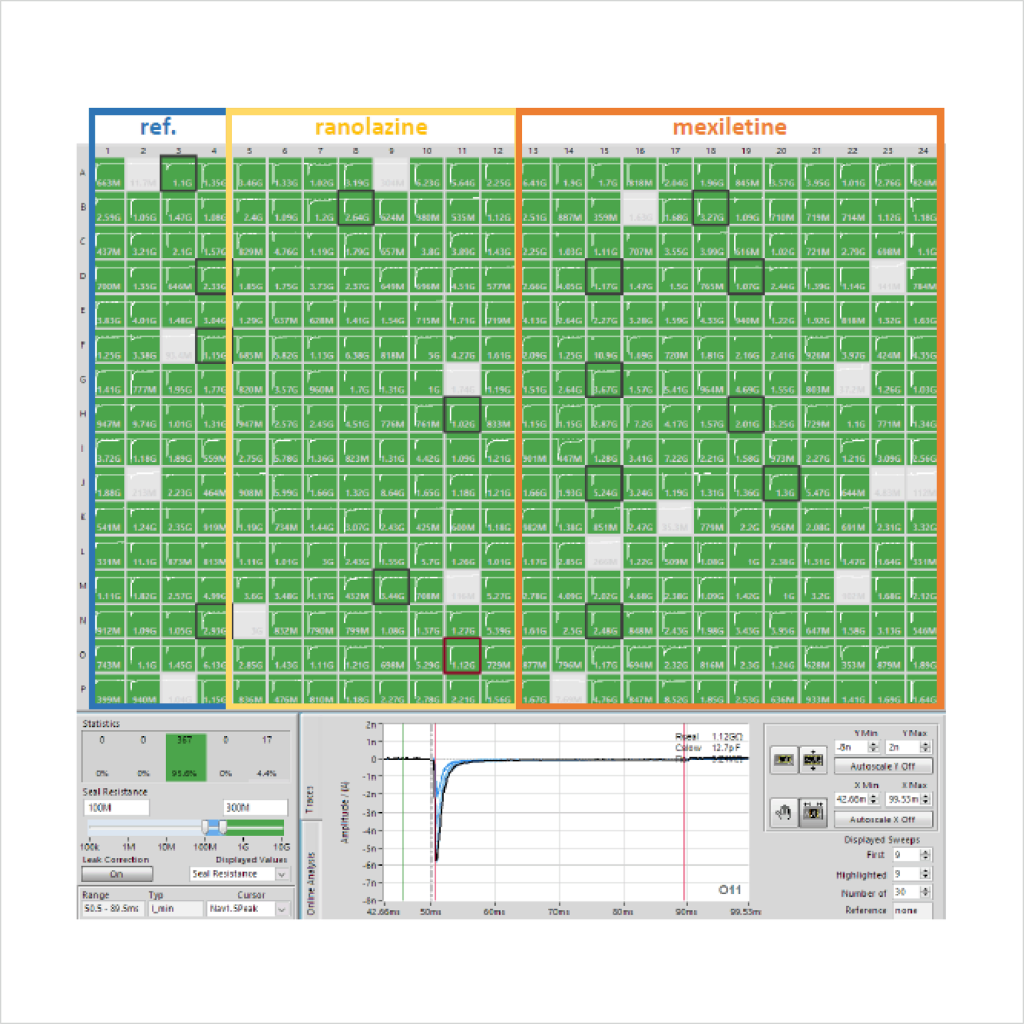
A major challenge to recording late openings of Nav1.5 channels is their small amplitude, which is negligible in WT channels (Fig. 2) but resolvable in ΔKPQ mutant channels using single-hole APC chips and of sufficient size for reliable biophysical and pharmacological assessment using multi-hole chips.
LQT3 ΔKPQ Nav1.5 currents exhibited steady state voltage-dependent activation (V1/2 -35 mV, left shifted compared to WT of -15 mV) and inactivation properties (Vh1/2 -75 mV) as expected using standard protocols⁵̕ ⁶.
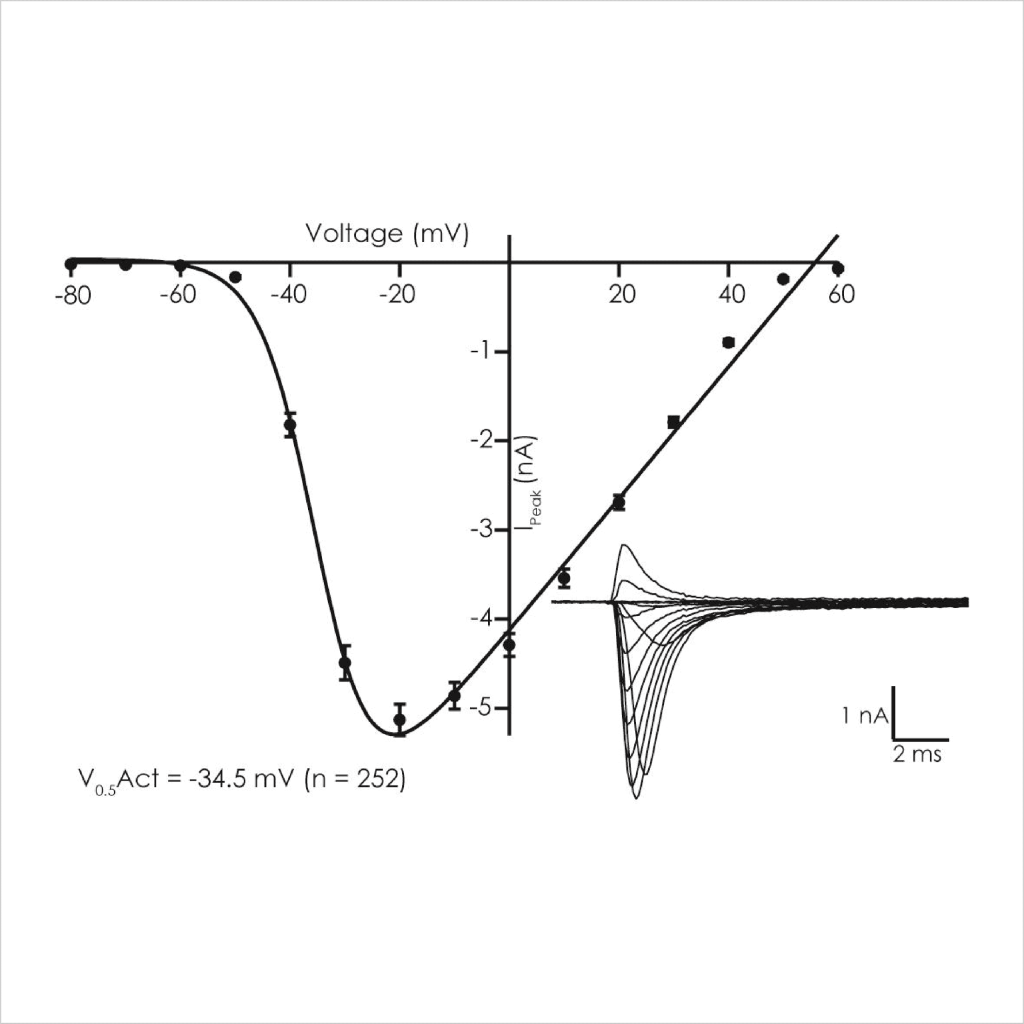
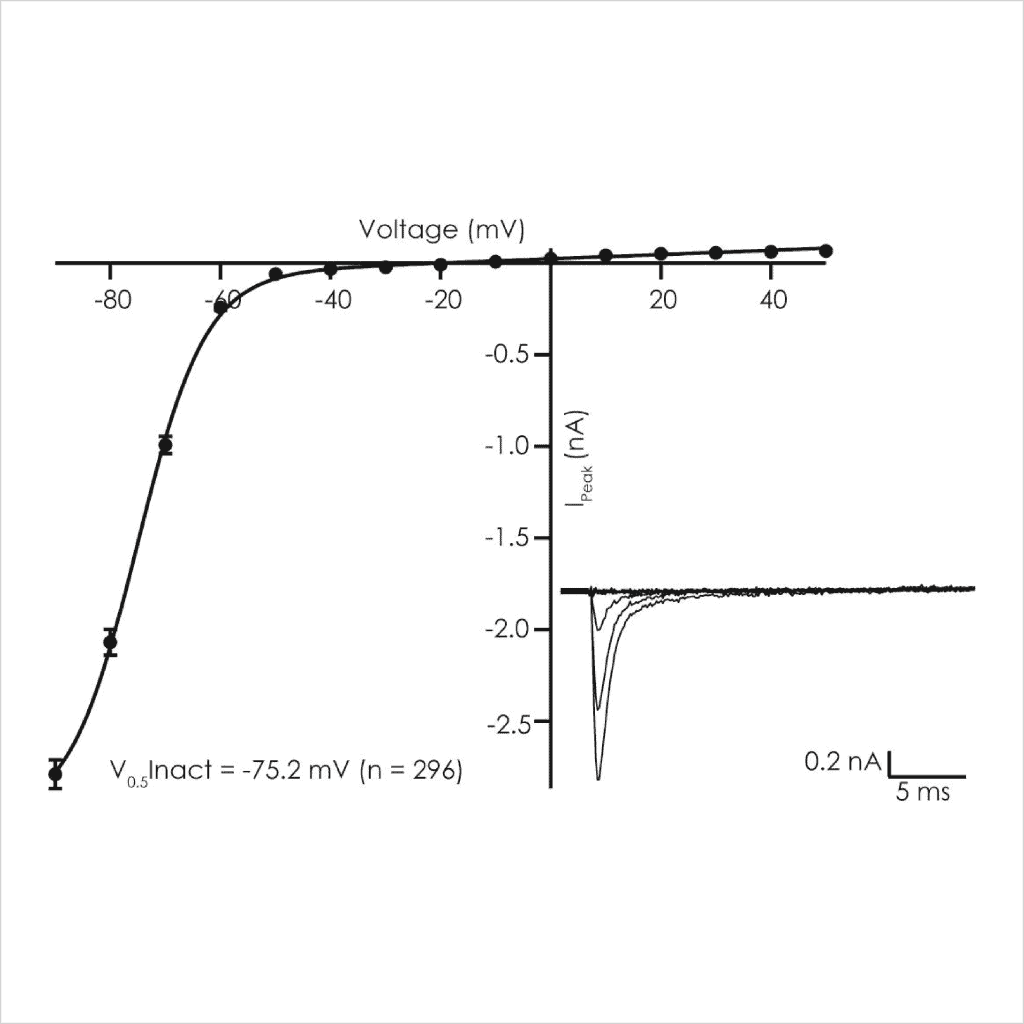
Figure 3: Voltage-dependent biophysical properties of ΔKPQ peak Nav1.5 currents. Activation (left) and steady-state inactivation (right) were determined using voltage steps delivered from a Vh of -100 mV.
Reference Pharmacology
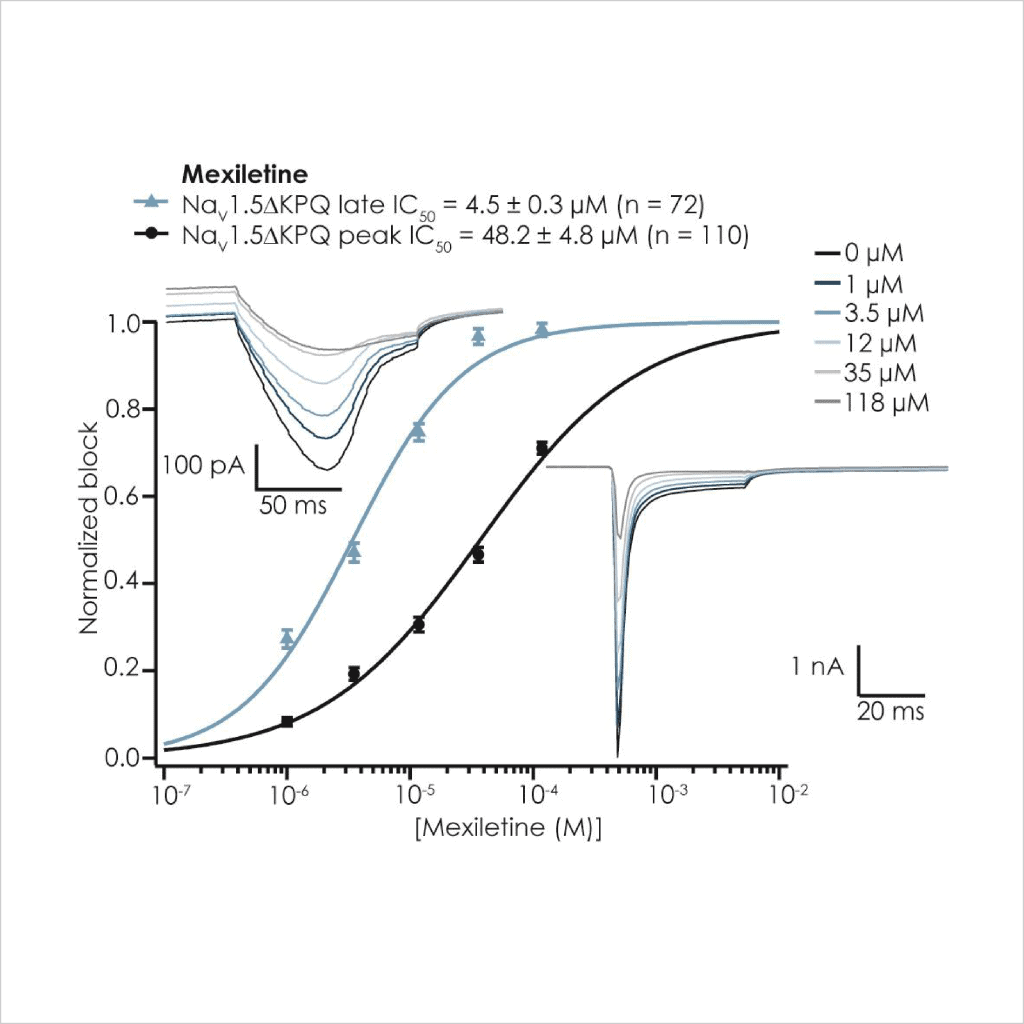
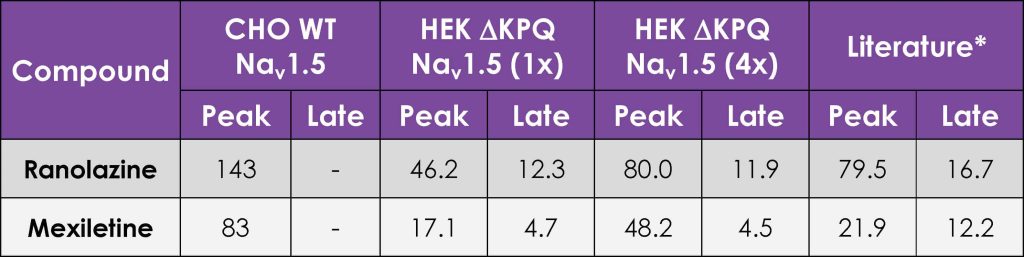
As there is little HTS APC data on ΔKPQ LQT3 late INa pharmacology it was important to test reference compounds and compare their potency to nonphysiological ATX-II toxin-activated channels, using the CiPA step-ramp protocol. We found that Ranolazine and Mexiletine inhibited mutant late INa with an IC50 of 17.5 µM (n = 74 wells) and 6.4 µM (n = 73 wells), respectively (Fig. 4), in good agreement with published values⁷. Peak WT and ΔKPQ currents were less sensitive than late INa components (Table 2).
Mexiletine
Ranolazine
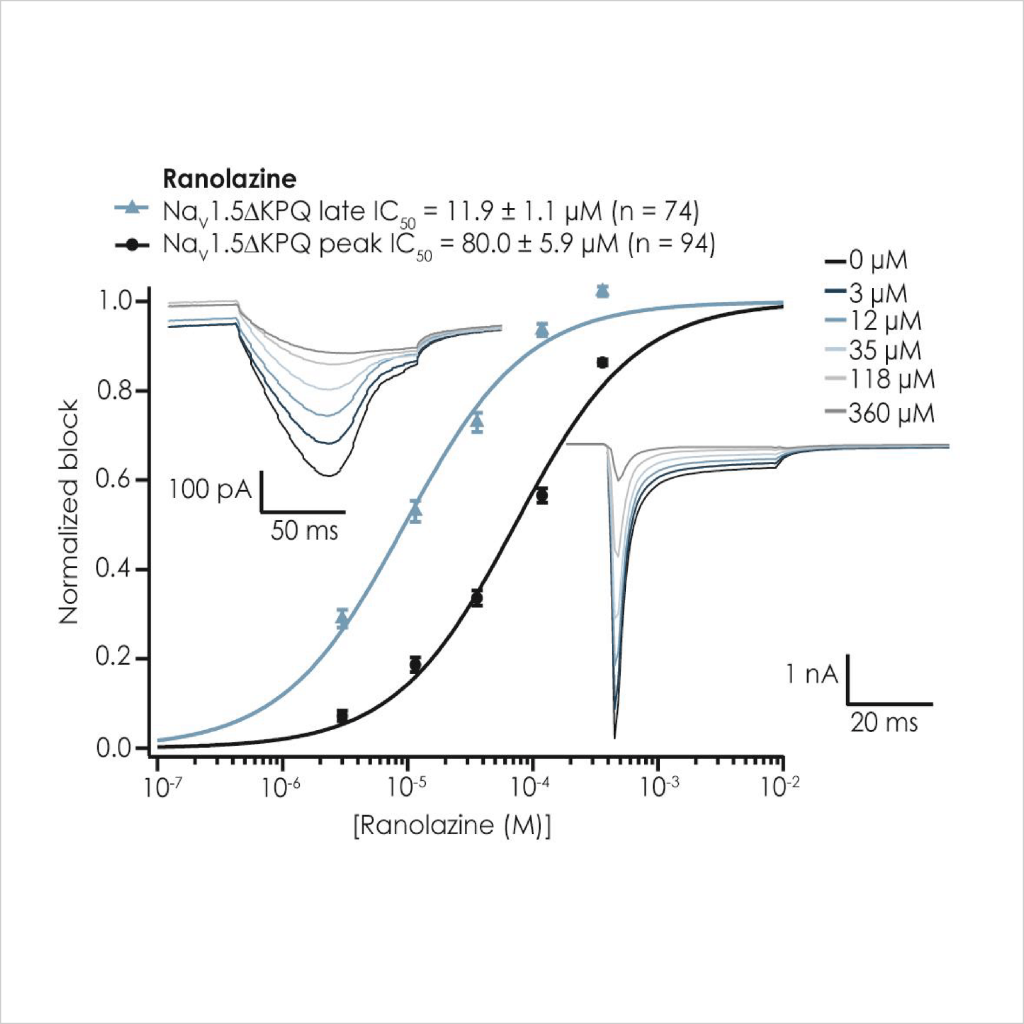
Figure 4: Pharmacological validation of ΔKPQ Nav1.5 currents. The inhibition of peak (black circles) and late current (blue triangles) is plotted against applied concentration of Mexiletine (left) and Ranolazine (right), and mean IC50 values are shown above each figure. Inset into each figure are late currents (upper left) evoked at the end of a step-ramp protocol, as well as peak and non-inactivating currents evoked using a step protocol, from 4x multi-hole chip recordings; note different amplitude axis range.
Conclusions
- Our collaboration was successful in using Metrion’s WT and ΔKPQ Nav1.5 cell lines and Nanion’s SyncroPatch 384i APC platform to design, optimise and validate a CiPA-ready HTS cardiac safety screening assay suitable for prediction of human clinical pro-arrhythmia risk.
- Gigaseal quality recordings were key to resolving the small late current openings of LQT3 mutant Nav1.5 channels without resorting to use of ATX-II.
- Nav1.5 ΔKPQ biophysical and pharmacological properties were reliably and efficiently determined for this optimised APC HTS cardiac safety assay.
References
- Wu et al., (2006) An Increase in Late Sodium Current Potentiates the Proarrhythmic Activities of Low-Risk QT-Prolonging Drugs in Female Rabbit Hearts. J Mol Pharm 316: 718-726.
- Li et al., (2018) Assessment of an In Silico Mechanistic Model for Proarrhythmia Risk Prediction Under the CiPA Initiative. Clin Pharm Therapeutics: 10.1002/cpt.1184.
- Crumb et al. (2016) An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods. 81: 251-262.
- Wang et al., (1995) SCN5A Mutations Associated with an Inherited Cardiac Arrhythmia, Long QT Syndrome. Cell 80: 805-811.
- Wang et al., (1996) Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. PNAS 93:13200-13205
- Bennett et al., (1995). Molecular mechanism for an inherited cardiac arrhythmia. Nature. 376: 683-685.
- Guo & Jenkinson (2019). J Pharm Tox Meth 99: 106575.

Let’s work together
What are your specific ion channel and assay needs?
If you have any questions or would like to discuss your specific assay requirements, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.
