ASIC Ion Channels: Key Regulators of Cellular Signaling
Introduction to ASIC Ion Channels:
ASICs (acid-sensing ion channels) are a family of ligand-gated ion channels that play a crucial role in cellular responses to acidic environments. They are involved in various physiological processes, including synaptic transmission, neuronal excitability, and pain sensation.
Five genes encode ASIC subunits in mammals and belong to the degenerin/epithelial sodium channels (DEG/ENaC) gene superfamily. In addition, several splicing variants produce several ASIC isoforms as listed in Table 1.
Three subunits combine to form homo- or heterotrimeric channels, which vary widely in proton sensitivity, activation/inactivation profiles, ion selectivity and pharmacology. Only ASIC2b and ASIC4 subunits cannot form homomeric channels.

Structure:
The prototypical ASIC subunits, such as ASIC1, ASIC2, and ASIC3, share a similar overall structure (Figure 1A), which mainly consists of a large extracellular region forming a funnel-like structure and two transmembrane domains (TM1 and TM2). The three TM2 domains form the pore of the ASIC heterotrimer (Figure 1B). The extracellular domain is responsible for H+-sensing and responding to a drop in extracellular pH. Current is carried mostly by Na+ ions and to a lesser extent by Ca2+ ions.
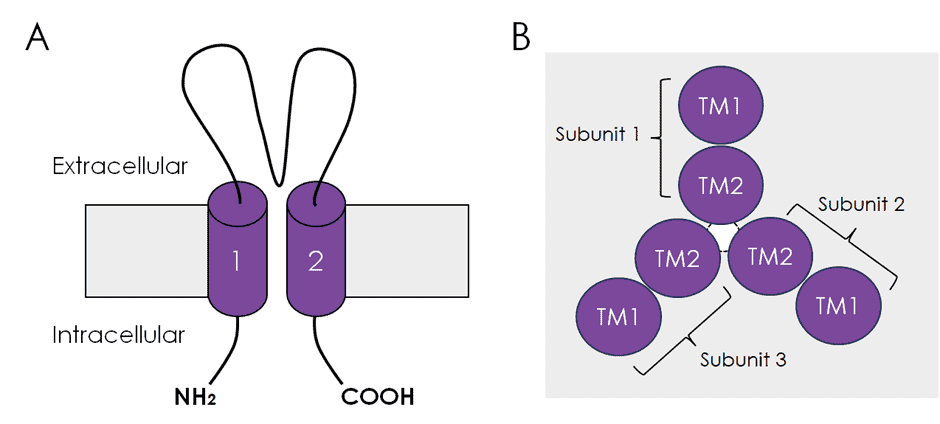
- ASIC subunits are composed of two transmembrane domains and a pre region.
- Functional ASIC channels are composed of three subunits and can form homo- or heterotrimers. The pore of the channels is lined by the second transmembrane domain of each subunit.
Physiological functions:
ASICs are primarily activated by extracellular acidification. When the surrounding pH drops, these channels open, which leads to the cell depolarisation.
At excitatory synapses of the CNS, it was proposed that ASICs are transiently activated by the decrease in pH in the synaptic cleft caused by the corelease of protons and glutamate in synaptic vesicles. While the possible acidification of the synaptic cleft milieu in normal physiological context is still debated, the postsynaptic location of ASICs and depolarizing effect of their activation suggest a role in excitatory postsynaptic signalling in many neurons. In agreement with this hypothesis, ASICs activation has been shown to modulate synaptic transmission and plasticity in the hippocampus.
In the PNS, ASICs are abundantly expressed in sensory neurons carrying noxious stimuli. ASIC3 subunits in particular play a key role in pH sensing for pain in nociceptive fibres and terminals.
Regulation of ASIC Channel Functions:
The activation and inactivation properties of ASICs are finely regulated, enabling precise control over cellular responses to changes in pH. Here are some key mechanisms involved in the regulation of ASICs:
- Protons: ASICs are highly sensitive to changes in extracellular pH. The resulting influx of cations causes the depolarization of the membrane and triggers intracellular pathways involved in the regulation of acidosis.
- Arachidonic acid: Arachidonic acid, a proinflammatory mediator can directly regulate ASICs activity.
- Modulation by Intracellular Signalling Pathways: Phosphorylation events mediated by protein kinases, such as PKC and PKA, can enhance or inhibit ASIC channel activity, contributing to the regulation of cellular responses to acidosis. Various modulations mediated by GPCRs and initiated by receptors to cannabinoid, 5-HT receptor or Neurokinin have been reported.
Pathological implications and therapeutic potential:
pH regulation is crucial for cellular homeostasis as cells face pH fluctuations from various sources. Metabolic processes and disorders, cellular stress, inflammation, and the microenvironment around tumours can all impact cellular pH. Cellular metabolism produces acidic byproducts, while inflammation and cellular stress lead to extracellular acidification. These different contexts contribute to the over-activation of ASICs in various diseases and disorders.
- Cancer: By sensing and responding to the acidic tumour microenvironment, ASIC1 and ASIC3 have been implicated in various cancer types, tumour progression and metastasis. For example, ASIC1 is overexpressed in pancreatic and breast cancer cells, and its upregulation was shown to promote tumour cell migration and invasion. ASIC3 is associated with prostate cancer, and its expression correlates with tumour stage and aggressiveness.
- Pain: ASIC channels, particularly ASIC1a and ASIC3, are abundantly expressed in sensory neurons as well as on non-neuronal cells in the muscles and joints and play a critical role in nociception. The acidosis associated with tissue injury or inflammation leads to the activation of ASIC channels. This contributes to the depolarization of sensory neurons and transmission of pain signals, making ASICs potential targets for analgesic therapies. Inhibiting ASIC channels has shown promise in alleviating pain in preclinical studies.
- Migraine: Migraine is a neurological disorder characterized by recurrent headaches, often accompanied by sensory disturbances. Studies have shown that ASIC channels are involved in migraine pathophysiology. Activation of ASIC channels in the trigeminal system, which is implicated in migraine, can trigger pain signalling, and promote the release of neuropeptides involved in headache generation.
- Epilepsy: Abnormal neuronal excitability is a hallmark of epilepsy. ASICs, particularly ASIC1a, have been implicated in modulating neuronal excitability and contributing to seizure generation. Increased expression of ASIC1a has been observed in animal models of epilepsy, and blocking ASIC1a has demonstrated anticonvulsant effects. Modulating ASICs may hold potential as a therapeutic strategy for epilepsy.
- Ischemic Stroke: Ischemic stroke occurs when blood flow to the brain is blocked, leading to tissue damage and neurological dysfunction. Acidosis is a common feature of ischemic stroke, and ASICs are known to be activated under acidic conditions. The activation of ASICs in ischemic conditions can contribute to neuronal injury and worsen stroke outcomes. Inhibiting ASICs has shown neuroprotective effects in experimental models of ischemic stroke.
Understanding the role of ASIC channels in disease states opens possibilities for developing targeted therapies.
Metrion’s Expertise in ASIC Channels:
As a leader in ion channel research and drug discovery, Metrion Biosciences offers comprehensive services related to ASIC ion channels. Our expertise and capabilities include:
- Electrophysiology Studies: We employ state-of-the-art electrophysiological techniques to characterize the properties and behaviour of ASICs. This enables us to assess their biophysical characteristics, voltage-dependence, and modulation by pharmacological agents.
- High-Throughput Screening: Our screening platforms enable efficient evaluation of compounds for their effects on ASICs. We assess compound potency, selectivity, and mode of action, aiding in the discovery of potential drug candidates targeting ASIC channels.
- Pharmacological Profiling: We provide detailed profiling services to evaluate the effects of compounds on different ASIC subunits. This comprehensive analysis helps to understand compound specificity, potential off-target effects, and therapeutic potential.
- Application Report [Metrion-App-Note-ASIC1a-ligand-gated-ion-channel-assay-v1.3.pdf (metrionbiosciences.com)]
- Validation Data [Frontiers | Development of ASIC1a ligand-gated ion channel drug screening assays across multiple automated patch clamp platforms (frontiersin.org)]
Conclusion:
ASICs are key regulators of cellular responses to acidic environments. Understanding their structure, function, and regulation provides insights into cellular and organ functions and their potential implications in disease states. Metrion Biosciences’ expertise in ASIC channels, along with our advanced technologies, contributes to the discovery and development of treatments targeting these ion channels.
Fun fact
An interesting compound called Psalmotoxin is derived from the venom of the Trinidad chevron tarantula (Psalmopoeus cambridgei). Psalmotoxin is a potent and selective inhibitor of ASIC1a channels, blocking their activity, which results in reducing pain perception. This allows the spider to immobilize and subdue its prey more effectively without triggering defensive reactions.
