
Specialist Cardiac Safety Screening
Reliably evaluate the proarrhythmic and cardiotoxic liabilities of your compounds
Our specialist team have been developing cardiac safety assays for over twenty years.
The cardiac safety screening assays we offer include:
- GLP hERG testing:
- Specialist GLP screening services against hERG using the conventional whole-cell patch-clamp technique.
- Generate GLP hERG data to support Investigational New Drug (IND) applications.
- Non-GLP hERG screening:
- Eliminate cardiac risk liability before lead development.
- VOLTA Assay: hiPSC cardiomyocyte model for early cardiac derisking:
- A powerful tool for safer and more efficient drug discovery.
- Clinical QTc/QRS prediction using hiPSC derived cardiomyocytes.
- Advantages of the hiPSC cardiomyocyte model.
- iPSC-derived cardiomyocyte screening:
- Successfully detect between compounds with low, medium and high proarrhythmic risk profiles.
- Assays for hERG, NaV1.5and CaV1.2.
- Dynamic hERG assay.
- Chronic cardiotoxicity assay: hiPSC derived cardiomyocytes:
- Measure the potential for test compounds to become trapped inside the hERG channel pore.
- Cardiac ion channel screening (CiPA):
- Provide an early assessment of potential off-target effects on cardiac ion channels by studying the effect of compounds on the CiPA ion channel panel.
- Evaluate the effect of compounds on contractility and viability of human iPSC-derived cardiomyocytes using an impedance platform.
- Exceptional ion channel electrophysiology and drug discovery expertise.
- A team of experienced cell biologists to create novel cell lines.
- High quality, cost-effective compound screening.
- Detailed characterisation of lead compounds in a range of high quality assays.
- Translational services including confirmation of efficacy in stem cell and other phenotypic models.
- Flexible approach that best suits your project and budget.
- Rapid turn-around times, reporting and data interpretation by highly experienced ion channel scientists.
- Derek Leishman (VP Translational and Quantitative Toxicology, Eli Lilly and Company).
- Steve Jenkinson (VP Drug Discovery and Safety, Metrion).
- CiPA hERG Milnes Kinetic Assay on Qpatch
- Nav1.5(late) Cardiac Safety Assay on Qpatch
- Nav1.5-ΔKPQ late INa current properties and pharmacology on the SyncroPatch 384i
- A systematic strategy for estimating hERG block potency and its implications in a new cardiac safety paradigm.
- Cross-site and cross-platform variability of automated patch clamp assessments of drug effects on human cardiac currents in recombinant cells.
- The Nav1.5 Late Current in WT and Nav1.5 ΔKPQ Mutant Channels: An Automated Patch Clamp LQT3 Electrophysiological Assay Comparison. Safety Pharmacology Society Virtual Meeting 2020.
- New CiPA Cardiac Ion Channel Cell Lines and Assays for In Vitro Proarrhythmia Risk Assessment. Japanese Safety Pharmacology Society Meeting, Tokyo, 2020
- Using new In Vitro Cardiac Ion Channel Assays and In Silico Models to Predict Proarrhythmia Risk with Automated Patch Clamp. Biophysical Society Annual Meeting, San Diego, 2020
- Development of an Impedance Based Screening Assay for Cardiac Safety and Cardiotoxicity Detection in Stem Cell derived Cardiomyocytes. Safety Pharmacology Society Annual Meeting, Barcelona, 2019
- Predicting Cardiac Proarrhythmic Risk Exclusively Using Automated Patch Clamp Data. Safety Pharmacology Society Annual Meeting, Barcelona, 2019
- New CiPA cardiac ion channel cell lines and assays for in vitro proarrhythmia risk assessment. Safety Pharmacology Society meeting, Washington DC, USA 2018.
- Assessment of human induced pluripotent stem cell-derived cardiomyocytes for evaluating drug-induced arrhythmias with multi-electrode array. Safety Pharmacology Society meeting, Washington DC, USA 2018.
- Refining in vitro QPatch cardiac ion channel QPatch and MEA iPSC cardiomyocyte assays for CiPA. SOT San Antonio 2018 poster Late Breaking 12: Safety Assessment: Pharmaceutical and Non-Pharmaceutical.
- CiPA update: Refining in vitro cardiac ion channel assays, in silico models and iPSC cardiomyocyte reagents for improved proarrhythmia risk prediction. SPS Vancouver September 2016 poster 0100.
- Human ventricular stem cell cardiomyocytes: validating in vitro assays and screening platforms for proarrhythmia risk prediction. SPS Vancouver September 2016 poster 0242.
- Human stem cell-derived cardiomyocytes: in vitro assays and screening platforms for exploring ventricular and atrial phenotypes. SPS Vancouver September 2016 poster 0247.
- Electrophysiological profiling of Axiogenesis CorV.4U iPSC-derived cardiomyocytes. Axiogenesis Workshop Cologne September 2016.
- Developing a package of in vitro human cardiac ion channel assays using automated patch clamp to predict clinical arrhythmia risk. SPS Prague September 2015.
- Presentation by Marc Rogers (Metrion CSO) at the June 2016 Sophion Ion Channel Modulation Symposium, Clare College, Cambridge (UK). CiPA update: in vitro cardiac ion channels screens, in silico models and stem cells iPS cardiomyocyte assays for pro-arrhythmia risk prediction.
- Presentation by Marc Rogers (Metrion CSO) at the September 2015 Safety Pharmacological Society, Prague. Using high quality HTS automated patch clamp data from human cardiac ion channels and in silico action potential modelling to cost-effectively predict QP prolongation and arrhythmia risk for CiPA.
- QPatch automated electrophysiology platform
- Patchliner automated electrophysiology
- Conventional manual patch clamp electrophysiology
- Plate-based impedance and microelectrode array techniques
- FlexStation plate-based imaging
Cardiac safety ion channel experts
By working with us you benefit from:
Our expertise in GLP hERG screening is demonstrated in the case study GLP hERG Assay Validation Following ICH E14/S7B 2022 Q&A Best Practice Guidelines.
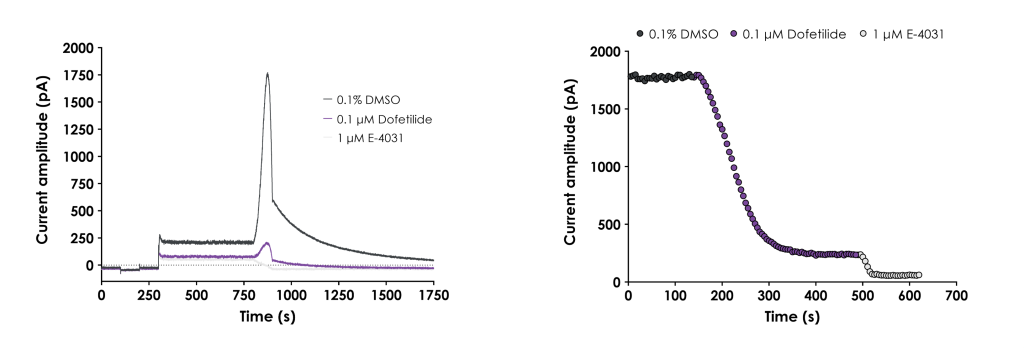
Webinar recording: In Vitro Assessment of Cardiac Risk in Drug Discovery
Download the recording of this webinar to learn how an hiPSC-CM model can help provide clear decision-making data for your project team that can avoid costly issues related to QTc and QRS cardiac liabilities in the clinic.
Recording includes presentations and Q&A:
Cardiac Safety Screening Resource Library
White Papers
Application notes
Publications
Posters
Flyers
Videos
Cardiac Safety Screening Technologies

Let’s work together
What are your specific ion channel screening requirements?
If you have any questions, or would like to discuss your project, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.

