GLP hERG Testing
Specialist GLP screening services against hERG using the conventional whole-cell patch-clamp technique
Why perform hERG screening?
The ICH S7A and ICH S7B guidelines provide regulatory guidance on performing safety pharmacology studies for human pharmaceuticals. Most Investigational New Drug (IND) applications for small molecules include pharmacological assessments against the human ether-à-go-go related gene (hERG) potassium channel, which should be conducted in compliance with GLP principles.
The human ether-à-go-go related gene (hERG) encodes the pore forming subunit of the rapidly activating delayed rectifier potassium current (IKr), which plays an important role in contributing to the repolarisation of ventricular cardiomyocytes.
The action potential duration of ventricular cardiomyocytes corresponds to the QT-interval of the electrocardiogram; therefore, inhibition of IKr can lead to prolongation of the cardiac action potential and the QT interval. Prolongation of the rate corrected QT interval beyond 440 ms is associated with an increased risk of arrythmias, such as the polymorphic ventricular tachycardia, Torsade de Pointes (TdP).
Several drugs have been withdrawn from the market, or had their use severely restricted, due to their proarrhythmic liability. Many of those drugs have been identified as hERG blockers and, consequently, ICH S7B guidelines were introduced that include guidance on evaluating the effect of new chemical entities against hERG using GLP principles. The implementation of hERG screening in drug discovery has had a significant contribution in reducing the development of proarrhythmic drugs.
GLP compliant hERG screening is mandatory under the ICH S7B guidelines, which states that in vitro IKr and in vivo QT assays described in Sections 2.3.1 and 2.3.2 when performed for regulatory submission should be conducted in compliance with good laboratory practice (GLP).
GLP hERG assay
We provide screening services against hERG using the conventional whole-cell patch-clamp technique. These services are performed in accordance with the FDA’s best practice guidelines and in compliance with:
1. UK GLP Regulations, SI 1999 No 3106; amendments, SI 2004 No. 994
2. OECD No 1 Principles on Good Laboratory Practice and Monographs.
The experiments are performed using the FDA’s recommended voltage protocol and experimental solutions at physiological temperature.
Concentration verification via chemical analysis of dosing formulations (Dose Formulation Analysis, DFA) is an obligatory requirement for GLP studies submitted in IND filings and is performed by our preferred GLP accredited partner.
Our services have been audited and approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA) and are performed in accordance with the Food and Drug Administration (FDA) best practice guidelines.
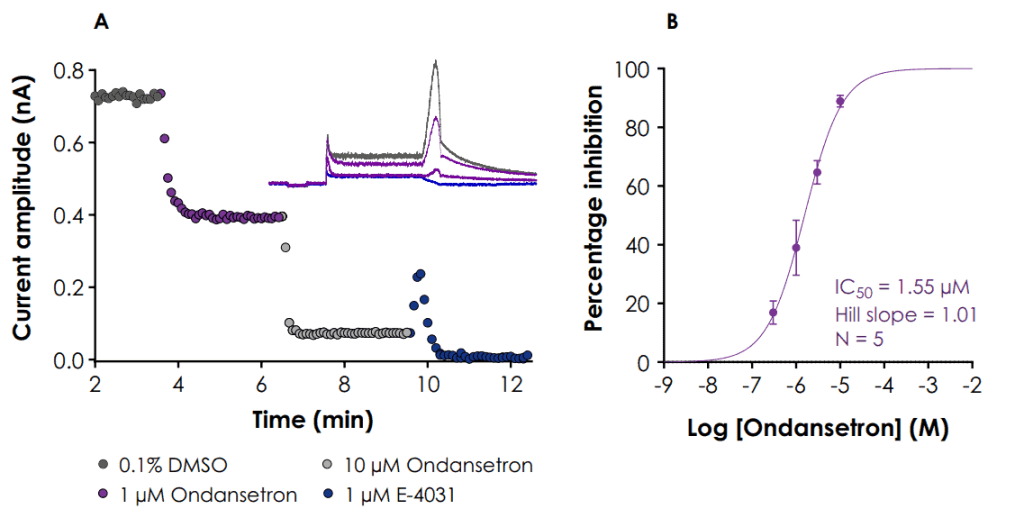
A. Graph showing current plotted against time for a representative cell treated with 1 and 10 μM ondansetron followed by 1 μM E-4031.
The inset figure shows representative current traces in 0.1% DMSO, ondansetron and E 4031.
B. Concentration response curve for ondansetron (data points ± SD)
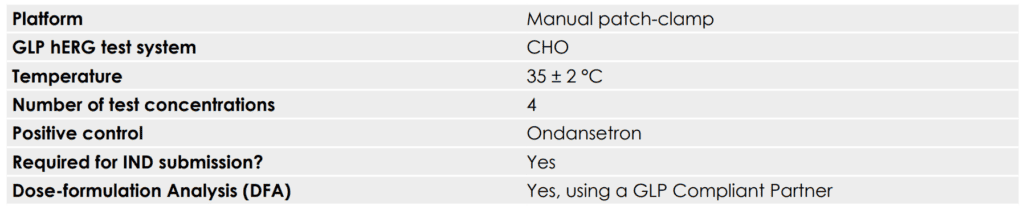
GLP hERG assay overview
Ion channel screening resource library
Publications
- Ion Channel Drug Discovery and Modern Medicine.
- Ion Channel Discovery – Partnering to Access Specialized Expertise.
- Recent advances in electrophysiology-based screening technology and the impact upon ion channel discovery research.
- Clathrodin, hymenidin and oroidin, and their synthetic analogues as inhibitors of the voltage-gated potassium channels.
- Novel K+ Channel Targets in Atrial Fibrillation Drug Development – Where Are We?
- Human Electrophysiological and Pharmacological Properties of XEN-D0101: A Novel Atrial-Selective Kv1.5/IKur Inhibitor.
Videos and Presentations
- Designing multiple assay protocols for ligand gated ion channels using the stacked-tip feature on the Patchliner and SP384i platforms
- The benefits of targeting ion channels for pain and some of the hurdles in developing successful ion channel modulators.
Posters
- Identification of Novel Scorpion Venom Peptide Inhibitors of the Kv1.3 Ion Channel and their Potential as Drug Discovery Leads for Human T-Cell Mediated Disease.
- The development of a set of novel small molecule inhibitors of the Kv1.3 ion channel.
- A drug discovery collaboration between Japanese pharma and a UK SME CRO successfully developed novel small molecule inhibitors of the Kv1.3 channel to treat autoimmune disease.
Application notes and resources
- ASIC1a Ligand Gated Ion Channel Assay (App Note)
- Investigating the correlation between thallium flux and automated patch-clamp for ion channel activators.
- Identification of novel ion-channel binders: TRPA1 antagonist case study.
- The development of a set of novel small molecule inhibitors of the Kv1.3 ion channel.
- Cross-site and cross-platform variability of automated patch clamp assessments of drug effects on human cardiac currents in recombinant cells.
- A systematic strategy for estimating hERG block potency and its implications in a new cardiac safety paradigm
- The Nav 1.5 Late Current in WT and Nav 1.5 ΔKPQ Mutant Channels: An Automated Patch Clamp LQT3 Electrophysiological Assay Comparison. Safety Pharmacology Society Virtual Meeting 2020.
- NaV1.5-ΔKPQ late INa current properties and pharmacology on the SyncroPatch 384i
- Recent advances in targeting ion channels to treat chronic pain.
- Marc Rogers (Metrion Director and Former CSO) takes part in a collaborative webinar with Nanion Technologies entitled “Validation and optimization of automated patch clamp voltage-gated Ca2+ channel assays”.
- Open access to the KCNQ channel: Retigabine and second generation M-current openers.
- Development of native and stem cell-derived electrophysiological assays for neurotoxicology screening and translational drug discovery
- Characterization of Endogenous Sodium Channels in the ND7-23 Neuroblastoma Cell Line: Implications for Use as a Heterologous Ion Channel Expression System Suitable for Automated Patch Clamp Screening.
- Optimising a difficult Nav1.8 cell line assay for automated patch clamp screening. Ion Channel Retreat, Vancouver, 2015
- Synthesis and biological evaluation of piperazine derivatives as novel isoform selective voltage-gated sodium (Nav) 1.3 channel modulators
- Action of Clathrodin and Analogues on Voltage-Gated Sodium Channels
- Novel state-dependent voltage-gated sodium channel modulators, based on marine alkaloids from Agelas sponges
- Ligand- and structure-based virtual screening for clathrodin-derived human voltage-gated sodium channel modulators
- The role of Nav1.7 in human nociceptors: insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients
- Assessment of human induced pluripotent stem cell-derived cardiomyocytes for evaluating drug-induced arrhythmias with multi-electrode array
- Development of an impedance based screening assay for cardiac safety and cardiotoxicity detection in stem cell derived cardiomyocytes
- Validation of an impedance-based phenotypic screening assay able to detect multiple mechanisms of chronic cardiotoxicity in human stem cell-derived cardiomyocytes
- Electrophysiological characterisation of Cellular Dynamics International ventricular iCell2 iPSC-derived cardiomyocytes
- Functional characterisation of human iPSC-derived atrial cardiomyocytes


Let’s work together
What are your specific GLP hERG Screening requirements?
If you have any questions, or would like to discuss your project, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.
