Investigating the correlation between thallium flux and automated patch-clamp for ion channel activators
Case Study
Case Study title
Investigating the correlation between thallium flux and automated patch-clamp for ion channel activators
Authors
David McCoull¹, Jonathan M Large¹, Alex Loucif², Raymond Tang², Ian Witton², Lewis Byrom¹, Edward Stevens², Jeff Jerman¹, Paul D Wright¹
- LifeArc, Accelerator Building, Open Innovation Campus, Stevenage, SG1 2FX, United Kingdom
- Metrion Biosciences Ltd, Riverside 3; Suite 1, Granta Park, Cambridge, CB21 6AD, United Kingdom
Overview
- Ion channels play a key role in regulating resting membrane potential and cell excitability and are attractive targets for therapeutic intervention.
- Thallium (Tl+) flux assays, which measure the flow of Tl+ through potassium channels, offer a high throughput method for the identification of potassium channel activators. However, these assays are a surrogate for channel function and it is important to have an appropriate panel of orthogonal and translational electrophysiology assays in place to confirm activity at the channel of interest.
- We used a Tl+ flux assay to screen a library of 100K compounds and identified 173 ‘hit’ compounds as potential activators of a K2P potassium channel. These compounds were then screened in an automated patch-clamp (APC) assay to confirm activity.
- Compounds with the highest activity in Tl+ flux were the most likely to confirm in automated patch-clamp, but the correlation between the two systems was not entirely uniform.
- Aim: to investigate the correlation between thallium flux and automated patch-clamp.
Methods
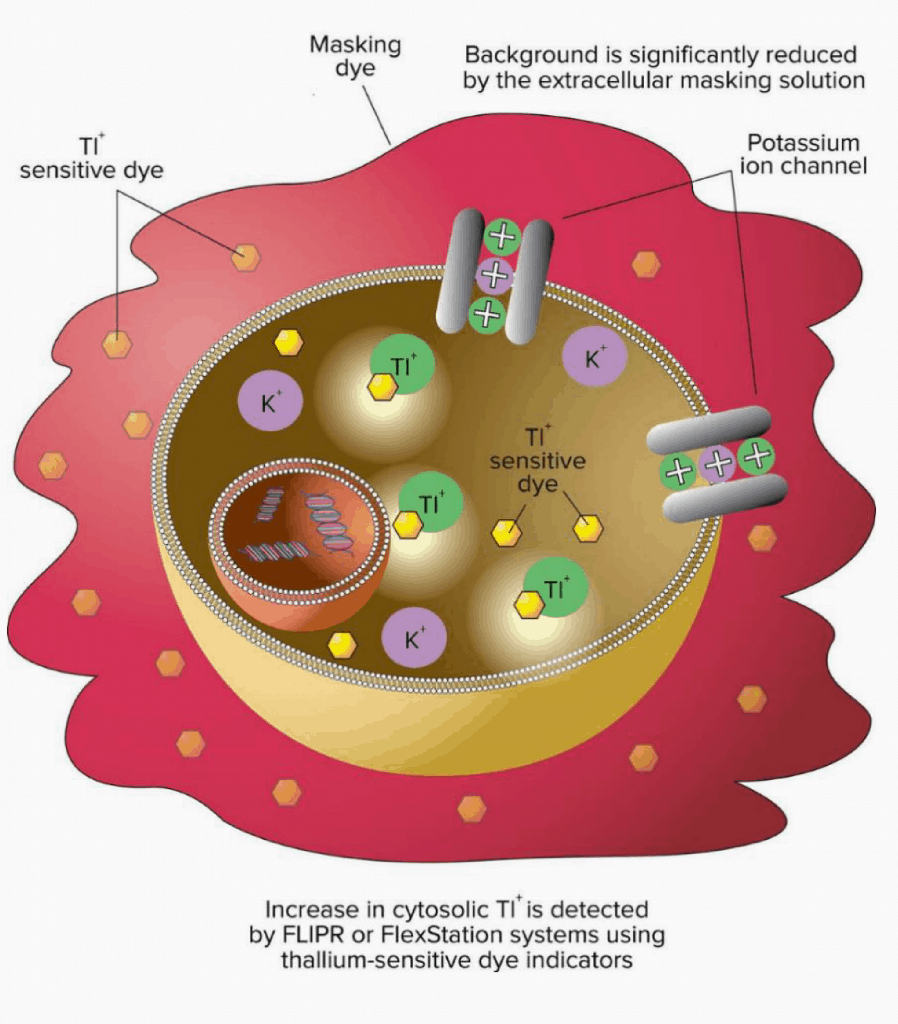
Figure 1: Schematic showing the use of intracellular thallium-sensitive dye to measure ion conduction through potassium channels as a screening assay to measure the effects of modulator compounds.
Thallium Flux
- FLIPR Potassium Assay Kit (Molecular Devices, USA) used according to manufacturers’ guidelines.
- Tl+ sensitive dye within the cell detects the flow of Tl+ through potassium channels.
- Typically, compounds are pre-incubated with cells for 30 minutes prior to Tl+ addition. Alternative pre-incubation periods were studied.
- 5-10K cells per well. Channel activity is measured as the rate of fluorescence increase following Tl+addition.
APC (QPatch)
- Cells in extracellular solution (ECS) move through a microfluidic system and suction pulls a single cell to the patch-clamp hole.
- After a gigaseal is formed, the membrane is ruptured, allowing whole-cell electrophysiological recordings to be performed.
- Test compounds are applied to the inlet wells. Continuous recording allows multiple concentrations / compounds to be applied to a single cell.
- Perforated-cell patch-clamp is a variant of traditional patch clamp, where pores are formed in the membrane, allowing electrical access but maintaining cytoplasmic constituents within the cell.
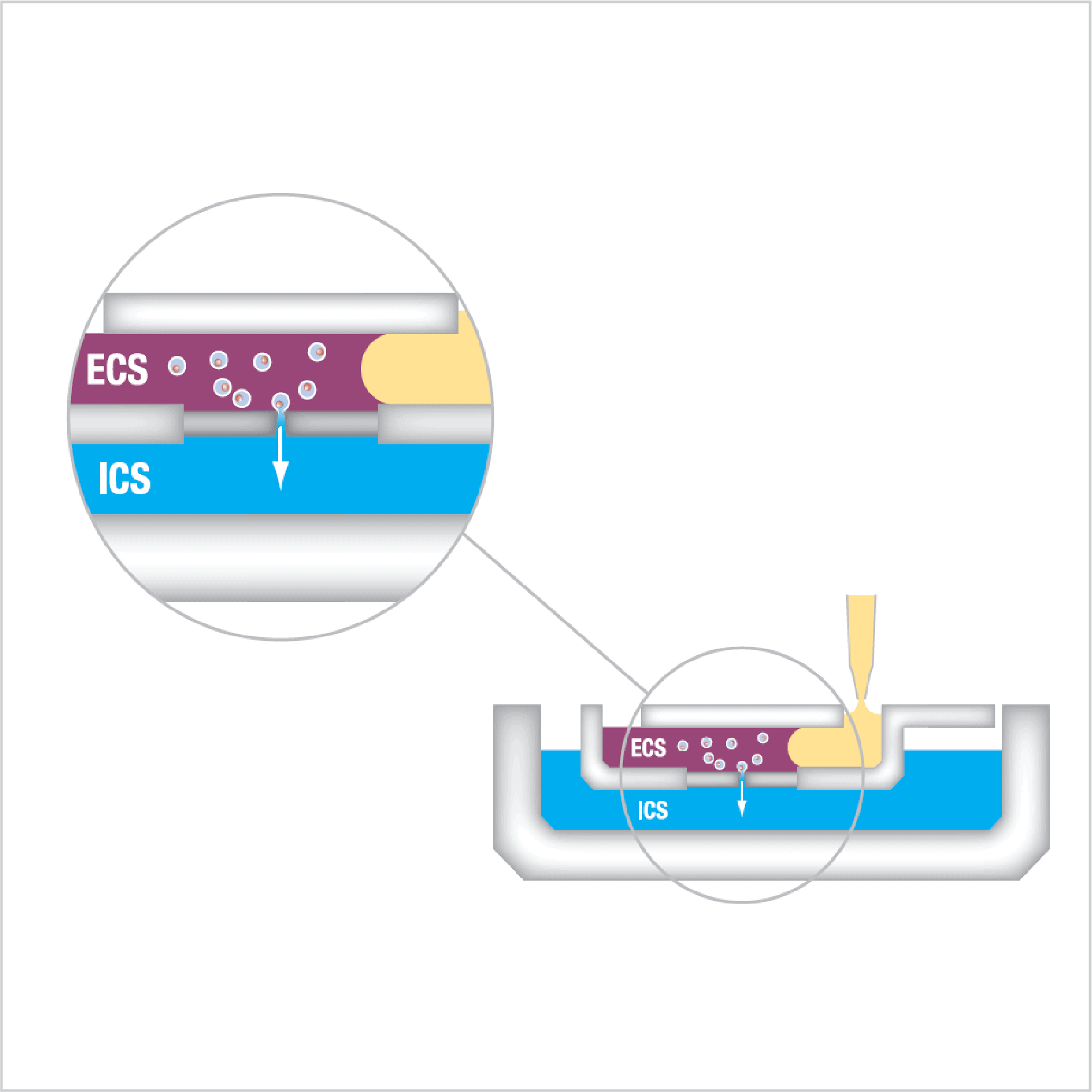
Figure 2: Schematic illustrating an automated patch clamp ‘chip’ well where cells are flowed over holes in a borosilicate substrate to obtain whole-cell access and enable patch clamp recordings of ion channel activity, and the effects of compounds added to each well (yellow).
Compound testing

- Dose response curves were prepared using either an acoustic (ECHO, Labcyte) or a tip-based (Biomek FX, Beckman Coulter) dispenser. Compounds were run in Tl+ flux assays with a 30 minute pre-incubation. Rate (15-28s) denotes the rate of Tl+ flux. (Data represent mean ± SD, n=2).
- A subset of compounds displayed a reduction in the magnitude of the response when they were handled on the acoustic dispenser, demonstrating that the liquid handling method can influence the response in Tl+ flux.
Automated and Manual Patch-clamp
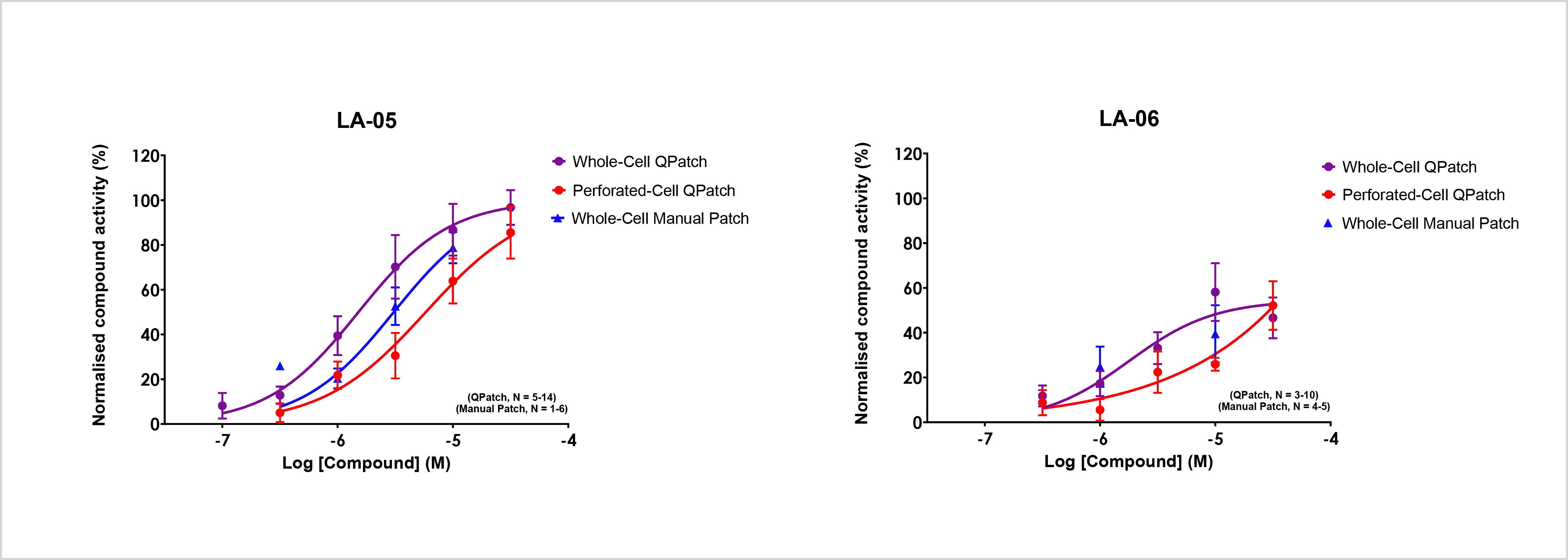
- Whole-cell, perforated-cell QPatch and whole-cell manual patch-clamp data for LA-05 and LA-06. (Data represent mean ± SEM, n≥1).
- Whole-cell and perforated-cell APC methods produce comparable results for both active and inactive compounds. Manual whole-cell patch-clamp (the gold standard patch-clamp technique), confirmed APC potency and efficacy data for active compounds.
Correlation Between Tl+ flux and APC

Figure 5: Comparison of K2P hit compound activity obtained by thallium flux HTS assay with hit validation activity from an APC (QPatch) platform.
- 173 compounds identified as ‘hits’ in a Tl+ flux screen of 100K compounds were screened in APC (QPatch).
- Plot shows correlation between Tl+ flux and APC data, coloured by activity in APC.
- Tl+ flux data represents fold-stimulation relative to DMSO control. APC data represents percentage of maximal channel activation.
- Compounds with the highest activity in Tl+ flux were the most likely to confirm in APC, but the correlation was not entirely uniform.
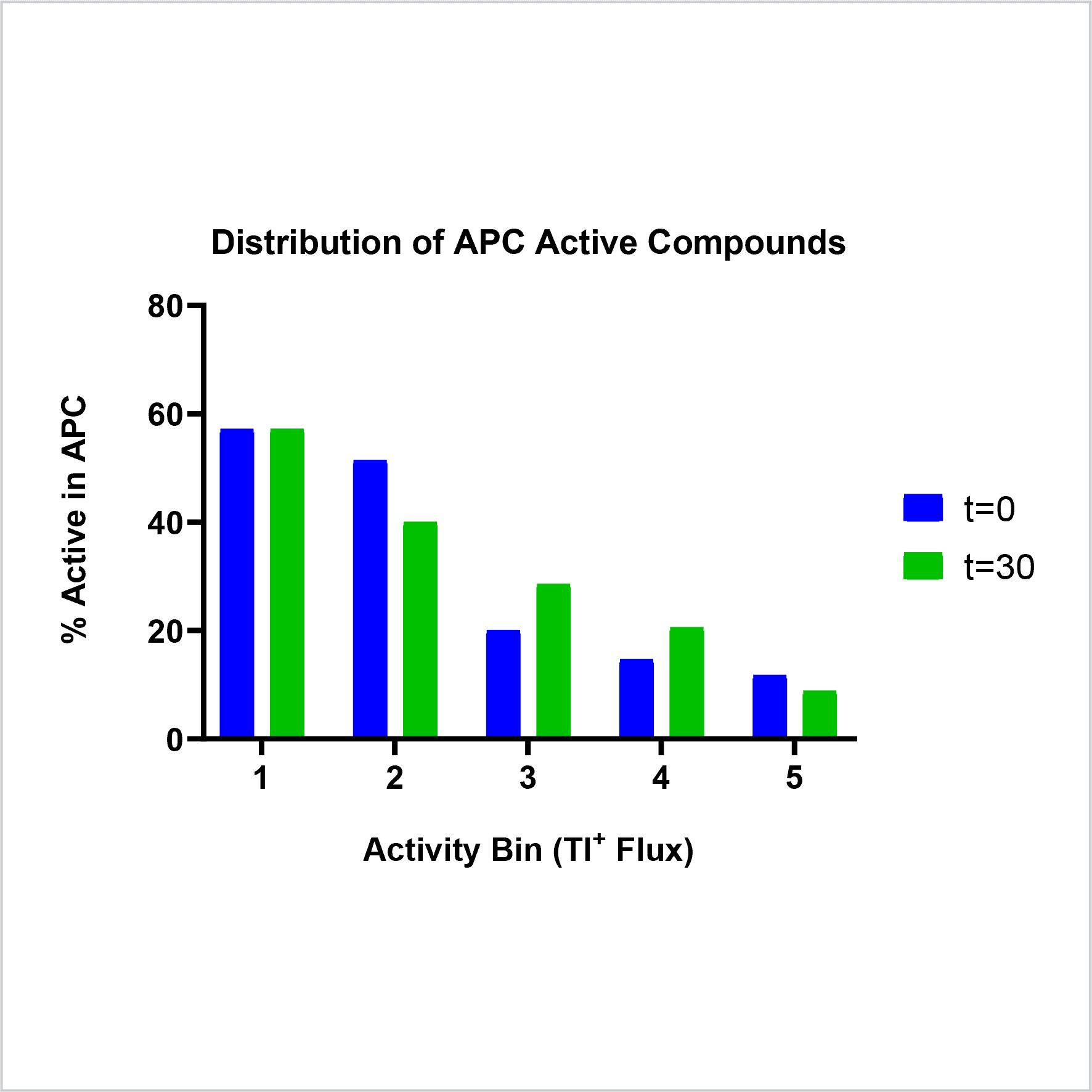
Figure 6: Correlation between thallium flux and APC hit compound activity is strongest for the most active compounds (bins 1-2) and for less active compounds pre-incubated in the thallium flux assay (green bars).
- Compounds were screened in Tl+ flux, with either a 30 minute (t=30) or no (t=0) pre-incubation.
- Compounds were then binned based on Tl+ flux activity; Bin 1 highest activity to Bin 5 lowest activity.
- For each bin, the percentage of compounds which were active in APC was then calculated.
- Data confirmed that compounds with the highest activity in Tl+ flux were the most likely to be active in APC.
- A subset of compounds showed higher activity at t=30 compared to t=0.
Compound Incubation Time
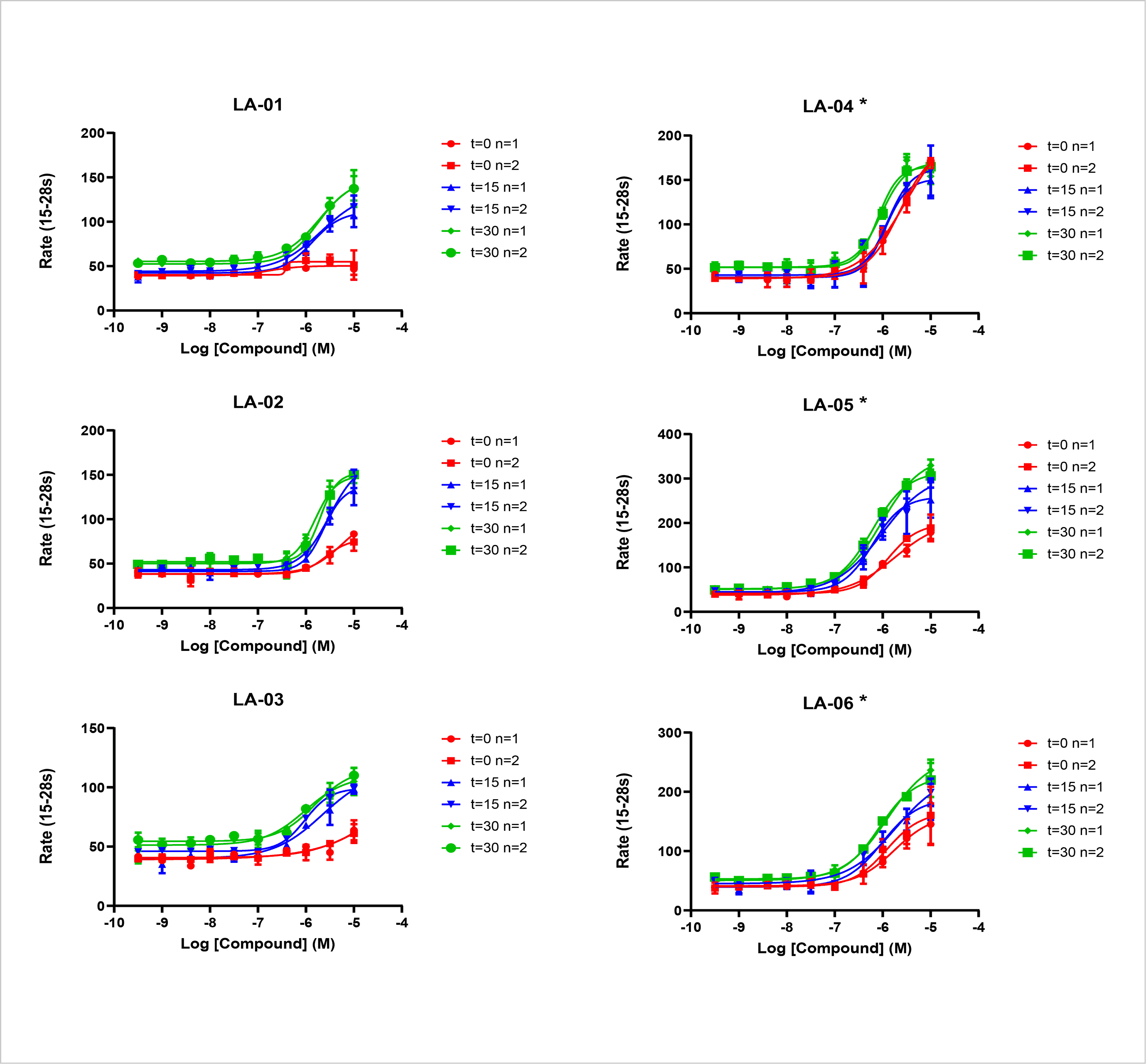
- Compounds were screened in dose response format in the Tl+ flux assay with either a 30 minute (t=30), a 15 minute (t=15) or no pre-incubation (t=0). Rate (15-28s) denotes the rate of Tl+ flux. (Data represent mean ± SD, n=2). * denotes compounds which were active in APC.
- A subset of compounds displayed a marked time dependency in the magnitude of their response. The magnitude of the response at t=0 may be indicative of the response in APC for some compounds.
Compound Properties
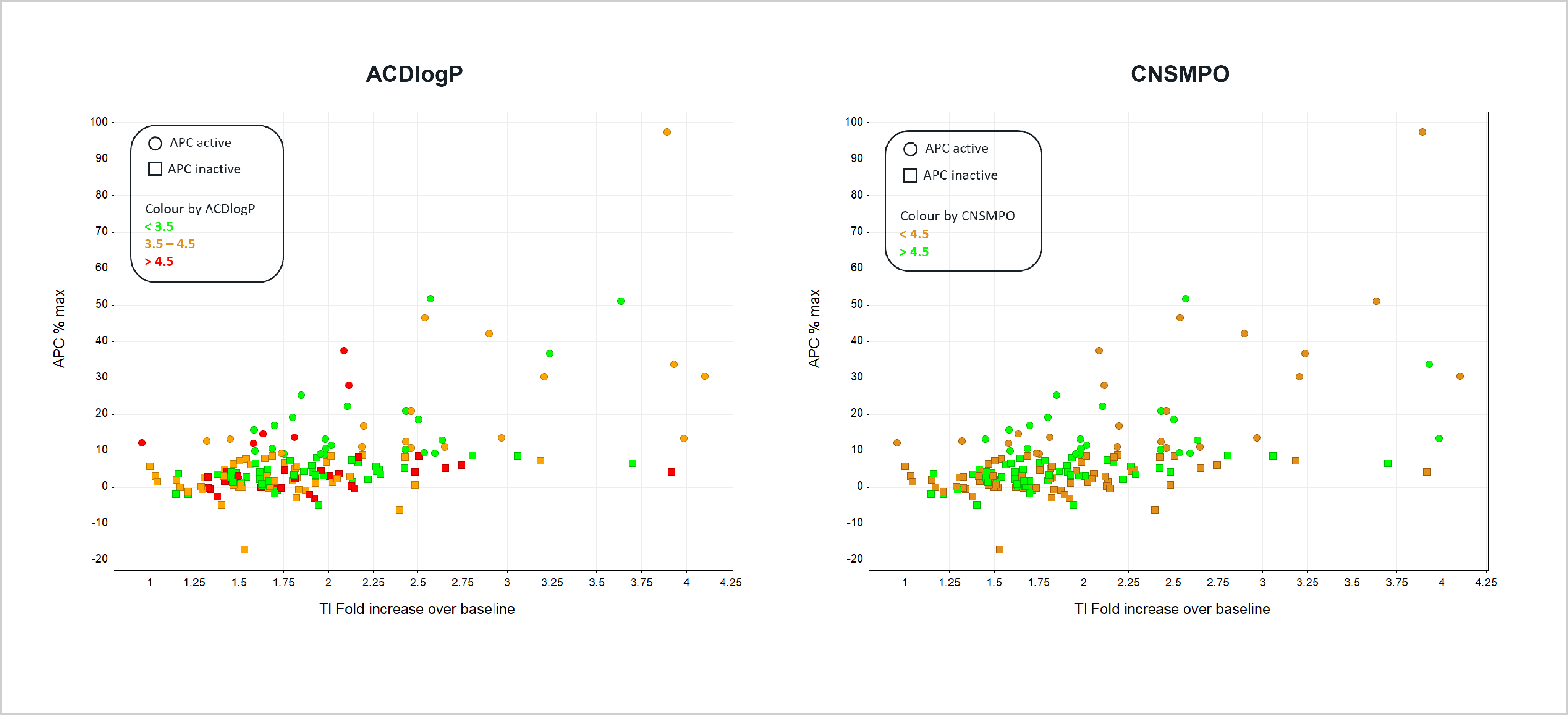
- Correlation between Tl+ flux and APC data, coloured by ACDlogP (left) or CNSMPO (right). ACDlogP is a measure of lipophilicity and CNSMPO a score for predicted CNS penetration.
- Neither metric could adequately explain the correlation between the two systems, but phys-chem properties of individual compounds may contribute to differential responses in the two systems.
Conclusions
- No single factor tested can adequately explain the correlation between the two systems.
- Compound incubation time and liquid handling method can influence the response in Tl+ flux.
- Looking at these factors in combination, prior to selecting compounds for electrophysiology, may enable us to prioritise screening outputs.

Let’s work together
What are your specific ion channel and assay needs?
If you have any questions or would like to discuss your specific assay requirements, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.
