Metrion Biosciences Application notes

Read our latest Application notes

CHO-KV 1.3 and current clamp using Qube 384: another perspective of ion channel behaviour
Introduction
KV 1.3 (KCNA3) is a voltage-gated potassium channel belonging to the Shaker related subfamily. KV 1.3 is best known for its role in activation of immune cells such as T Lymphocytes and microglial cells, where high expression is found. However more recently, KV 1.3 channels have also been demonstrated to be present in the mitochondria and nuclei of cancer cells.
Abstract
The KV 1.3 channel displays fast activation kinetics and rapid C-type inactivation. The biophysical properties of KV 1.3 give rise to a steady-state conductance over a small range of voltages (the window current). In T cells, KV 1.3 regulates RMP at the voltage range of this window current region.
Combining both voltage clamp and current clamp recording techniques allows a better understanding of the physiological effects of compounds targeting ion channels. In this report, we investigated pharmacological effects of KV 1.3 inhibitotors on KV 1.3 currents alongside RMP of a CHO- KV 1.3 cell line with our automated patch clamp system, the Qube 384. Current clamp mode significantly expands the automated electrophysiology toolset for understanding pharmacological and physiological effects of ion channel modulators.
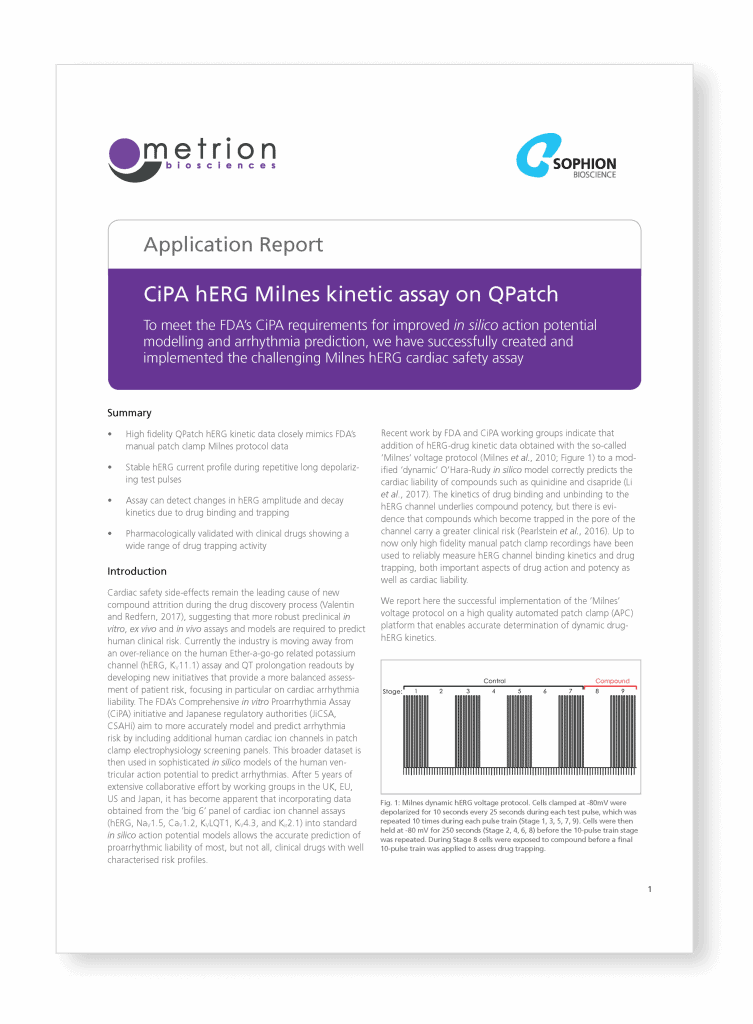
CiPA hERG Milnes kinetic assay on QPatch
Introduction
To meet the FDA’s CiPA requirements for improved in silico action potential modelling and arrhythmia prediction, we have successfully created and implemented the challenging Milnes hERG cardiac safety assay.
Abstract
Cardiac safety side-effects remain the leading cause of new compound attrition during the drug discovery process (Valentin and Redfern, 2017), suggesting that more robust preclinical in vitro, ex vivo and in vivo assays and models are required to predict human clinical risk. Currently the industry is moving away from an over-reliance on the human Ether-a-go-go related potassium channel (hERG, KV11.1) assay and QT prolongation readouts by developing new initiatives that provide a more balanced assessment of patient risk, focusing in particular on cardiac arrhythmia liability.
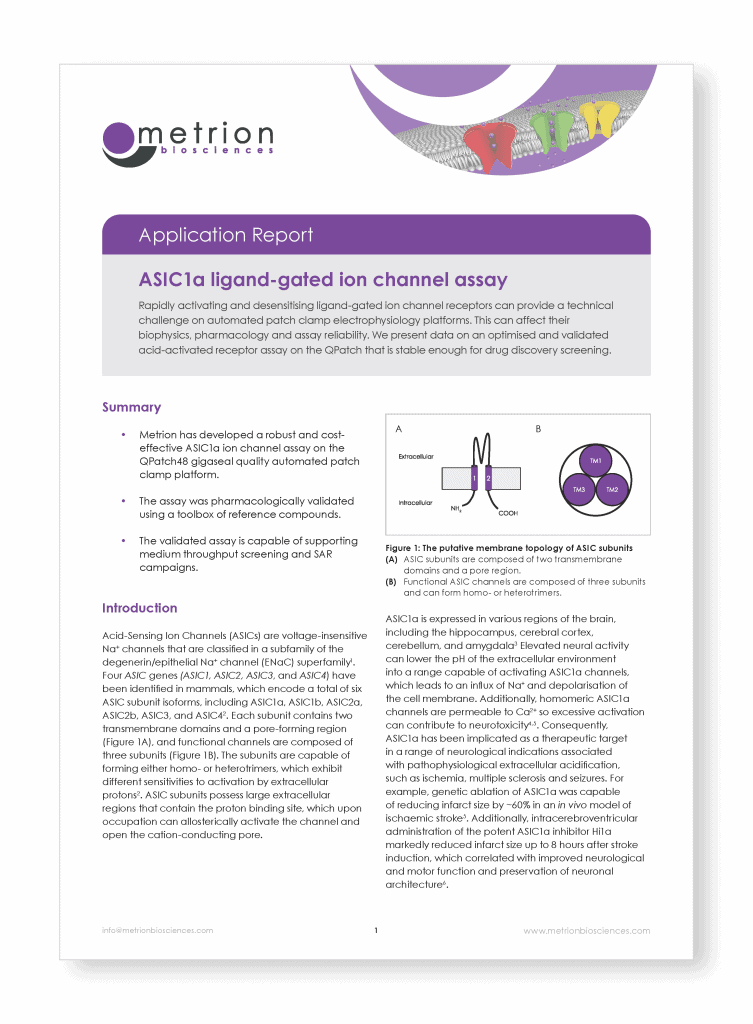
ASIC1a ligand-gated ion channel assay
Introduction
Rapidly activating and desensitising ligand-gated ion channel receptors can provide a technical challenge on automated patch clamp electrophysiology platforms. This can affect their biophysics, pharmacology and assay reliability. We present data on an optimised and validated acid-activated receptor assay on the QPatch that is stable enough for drug discovery screening.
Abstract
Acid-Sensing Ion Channels (ASICs) are voltage-insensitive Na+ channels that are classified in a subfamily of the degenerin/epithelial Na+ channel (ENaC) superfamily1. Four ASIC genes (ASIC1, ASIC2, ASIC3, and ASIC4) have been identified in mammals, which encode a total of six ASIC subunit isoforms, including ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC42.

NaV1.5(Late) cardiac safety assay on QPatch
Introduction
As an alternative to standard pharmacological procedures for NaV1.5(Late) assays, we present a more reliable and accurate NaV1.5(Late) assay on QPatch that removes the requirement for activators like veratridine and ATX-II and delivers improved cardiac safety screening reliability and cost.
Abstract
Cardiac safety side-effects remain the major cause of new compound attrition during the drug discovery process. This suggests that more robust preclinical in vitro, ex vivo and in vivo assays and models are required to predict clinical risk in humans. Currently the industry is moving away from an over-reliance on the human ether-a-go-go related gene (hERG) potassium channel (also known as KV11.1) and QT prolongation readouts by developing new initiatives that provide a more balanced assessment of patient risk, focusing in particular on proarrhythmic liability.

NaV1.5-ΔKPQ late INa current properties and pharmacology on the SyncroPatch 384i
Introduction
One aim of the Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative is to improve drug safety testing in pre-clinical development by evaluating the proarrhythmic risk of a compound. Validation studies confirm that testing the effect of compounds on an increased number of human cardiac ion channel currents including INa (NaV1.5 peak and late current) as well as IKr (hERG) leads to improved prediction of their clinical risk.
Abstract
The cardiac late Na current (late INa) generates persistent currents throughout the plateau phase of the cardiac action potential. Several mutations in the SCN5A gene cause a form of hereditary long QT syndrome (LQT3)1-3. The ΔKPQ mutation deletes residues Lys 1505, Pro 1506 and Gln 1507, resulting in a sustained, non-inactivating current during long (over 50 ms) depolarizations1,2. This sustained current causes prolongation of the action potential which can result in fatal ventricular arrhythmias such as Torsade de Pointes (TdP)1.
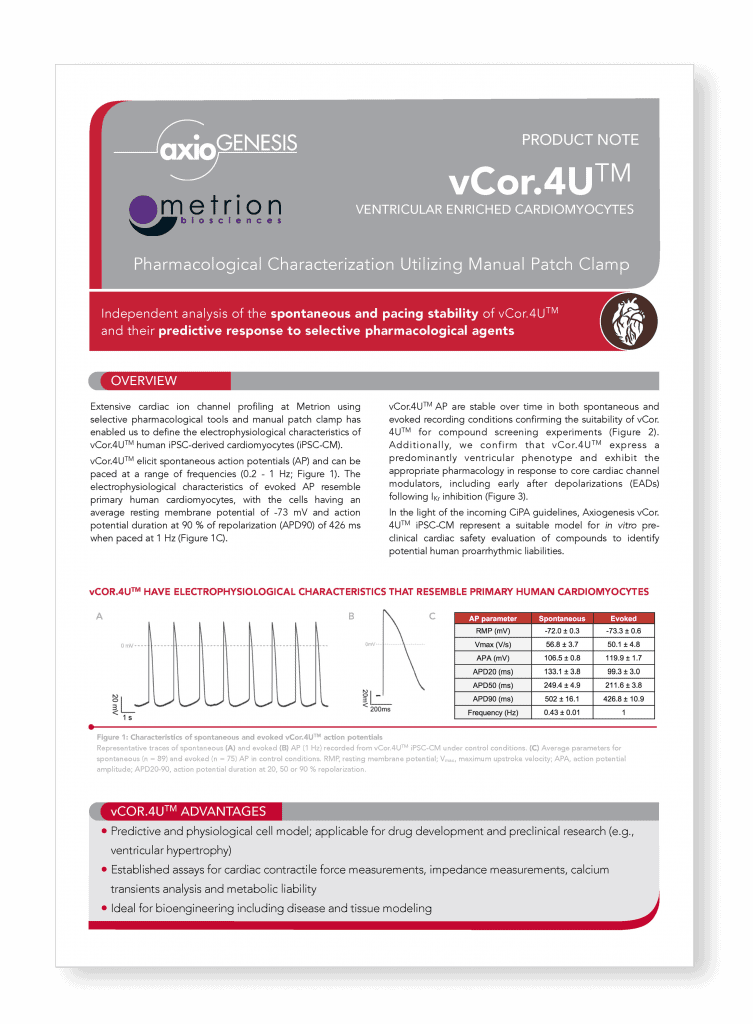
vCor.4U™ Ventricular Enriched Cardiomyocytes: Pharmacological Characterization Utilizing Manual Patch Clamp
Introduction
Metrion Biosciences independent analysis of the spontaneous and pacing stability of vCor.4UTM and their predictive response to selective pharmacological agents.
Abstract
Extensive cardiac ion channel profiling at Metrion using selective pharmacological tools and manual patch clamp has enabled us to define the electrophysiological characteristics of vCor.4UTM human iPSC-derived cardiomyocytes (iPSC-CM). vCor.4UTM elicit spontaneous action potentials (AP) and can be paced at a range of frequencies (0.2 – 1 Hz). The electrophysiological characteristics of evoked AP resemble primary human cardiomyocytes, with the cells having an
average resting membrane potential of -73 mV and action potential duration at 90 % of repolarization (APD90) of 426 ms when paced at 1 Hz.

Let’s work together
What are your specific ion channel and assay needs?
If you have any questions or would like to discuss your specific assay requirements, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.
