Metrion Biosciences Posters

View our Posters
2024
GLP hERG Assay Validation following ICH E14/ S7B 2022 Q&A Best Practice Guidelines
Metrion Biosciences, February 2024
Introduction
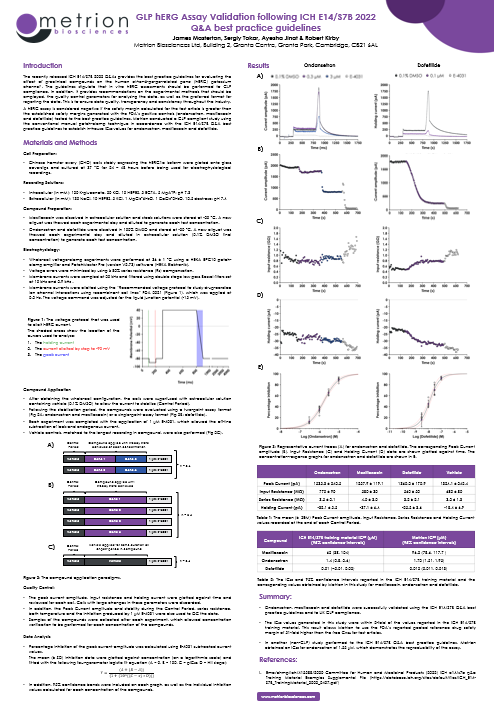
The recently released ICH E14/S7B 2022 Q&As provides the best practice guidelines for evaluating the effect of preclinical compounds on the human ether-à-go-go-related gene (hERG) potassium channel1. The guidelines stipulate that in vitro hERG assessments should be performed to GLP compliance. In addition, it provides recommendations on the experimental methods that should be employed, the quality control parameters for analysing the data, as well as the preferred format for reporting the data. This is to ensure data quality, transparency and consistency throughout the industry. A hERG assay is considered negative if the safety margin calculated for the test article is greater than the established safety margins generated with the FDA’s positive controls (ondansetron, moxifloxacin and dofetilide) tested to the best practice guidelines. Metrion conducted a GLP compliant study using the conventional manual patch-clamp technique in accordance with the ICH E14/S7B Q&A best practice guidelines to establish in-house IC50 values for ondansetron, moxifloxacin and dofetilide.
2023
Development of a manual patch-clamp assay to characterise native endo-lysosomal ion channels
Metrion Biosciences, June 2023
Introduction
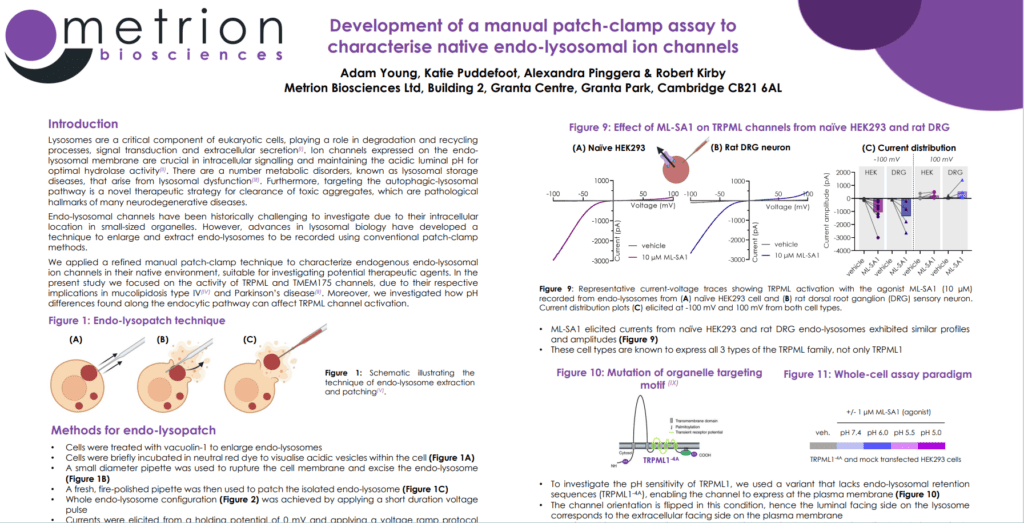
Lysosomes are a critical component of eukaryotic cells, playing a role in degradation and recycling processes, signal transduction and extracellular secretion(I). Ion channels expressed on the endo-lysosomal membrane are crucial in intracellular signalling and maintaining the acidic luminal pH for optimal hydrolase activity(II). There are a number metabolic disorders, known as lysosomal storage diseases, that arise from lysosomal dysfunction(III).
Furthermore, targeting the autophagic-lysosomal pathway is a novel therapeutic strategy for clearance of toxic aggregates, which are pathological hallmarks of many neurodegenerative diseases. Endo-lysosomal channels have been historically challenging to investigate due to their intracellular location in small-sized organelles. However, advances in lysosomal biology have developed a technique to enlarge and extract endo-lysosomes to be recorded using conventional patch-clamp methods.
We applied a refined manual patch-clamp technique to characterize endogenous endo-lysosomal ion channels in their native environment, suitable for investigating potential therapeutic agents. In the present study we focused on the activity of TRPML and TMEM175 channels, due to their respective implications in mucolipidosis type IV(IV) and Parkinson’s disease(II). Moreover, we investigated how pH differences found along the endocytic pathway can affect TRPML channel activation.
Rescue of defective CFTR (dF508) is enhanced by targeting the ubiquitin proteasomal system
Metrion Biosciences and Entact Bio, April 2023
Introduction
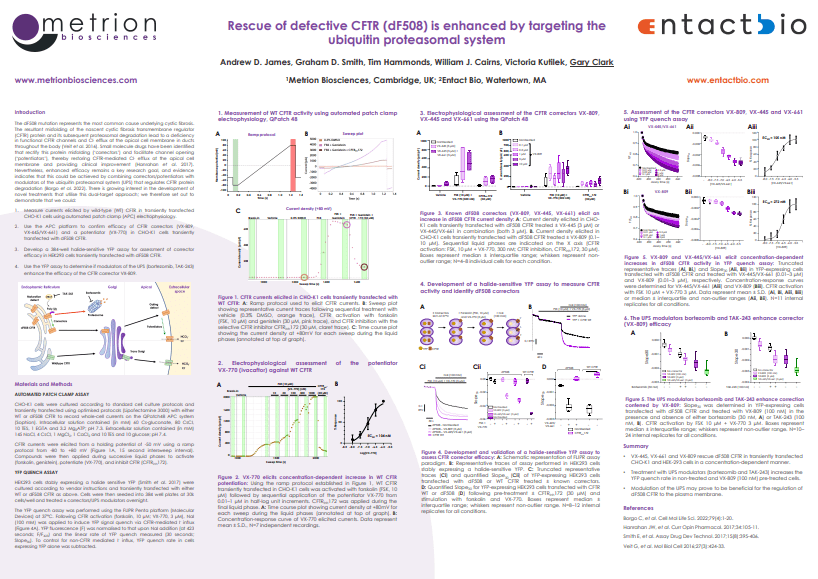
The dF508 mutation represents the most common cause underlying cystic fibrosis. The resultant misfolding of the nascent cystic fibrosis transmembrane regulator (CFTR) protein and its subsequent proteasomal degradation lead to a deficiency in functional CFTR channels and Cl- efflux at the apical cell membrane in ducts throughout the body (Veit et al. 2016). Small molecule drugs have been identified that rectify this protein misfolding (‘correctors’) and facilitate channel opening (‘potentiators’), thereby restoring CFTR-mediated Cl- efflux at the apical cell membrane and providing clinical improvement (Hanrahan et al. 2017).
Nevertheless, enhanced efficacy remains a key research goal, and evidence indicates that this could be achieved by combining correctors/potentiators with modulators of the ubiquitin proteasomal system (UPS) that regulates CFTR protein degradation (Borgo et al. 2022). There is growing interest in the development of novel treatments that utilise this dual-target approach; we therefore set out to demonstrate that we could:
- Measure currents elicited by wild-type (WT) CFTR in transiently transfected
CHO-K1 cells using automated patch clamp (APC) electrophysiology. - Use the APC platform to confirm efficacy of CFTR correctors (VX-809,
VX-445/VX-661) and a potentiator (VX-770) in CHO-K1 cells transiently
transfected with dF508 CFTR. - Develop a 384-well halide-sensitive YFP assay for assessment of corrector
efficacy in HEK293 cells transiently transfected with dF508 CFTR. - Use the YFP assay to determine if modulators of the UPS (bortezomib, TAK-243)
enhance the efficacy of the CFTR corrector VX-809.
Development and validation of a Qube automated patch clamp hERG assay at physiological temperatures
Japanese Safety Pharmacology Society Annual Meeting, February 2023
Introduction
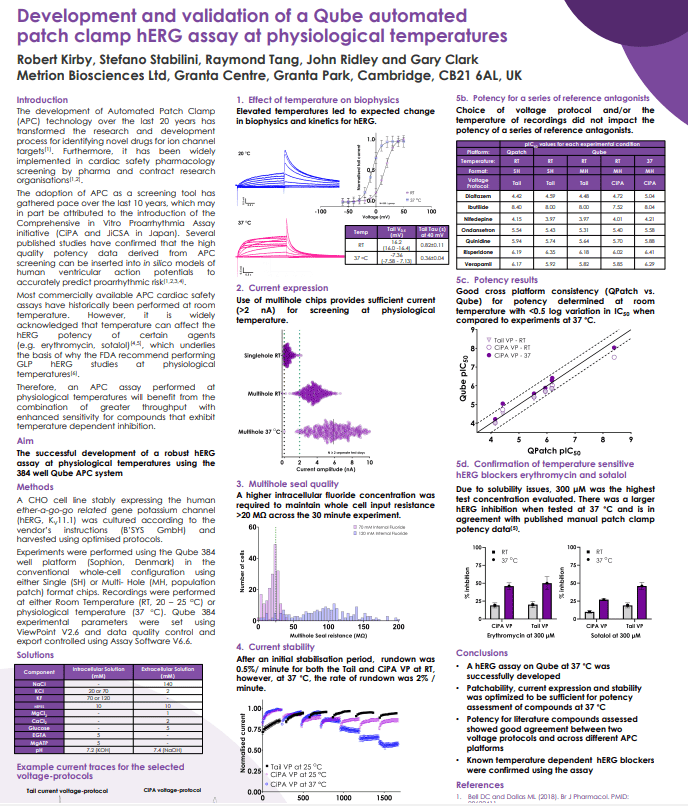
The development of Automated Patch Clamp (APC) technology over the last 20 years has
transformed the research and development process for identifying novel drugs for ion channel targets. Furthermore, it has been widely implemented in cardiac safety pharmacology screening by pharma and contract research organisations.
The adoption of APC as a screening tool has gathered pace over the last 10 years, which may
in part be attributed to the introduction of the Comprehensive in Vitro Proarrhythmia Assay
initiative (CiPA and JiCSA in Japan). Several published studies have confirmed that the high
quality potency data derived from APC screening can be inserted into in silico models of human ventricular action potentials to accurately predict proarrhythmic risk.
Most commercially available APC cardiac safety assays have historically been performed at room temperature. However, it is widely acknowledged that temperature can affect the hERG potency of certain agents (e.g. erythromycin, sotalol), which underlies the basis of why the FDA recommend performing GLP hERG studies at physiological temperatures. Therefore, an APC assay performed at physiological temperatures will benefit from the combination of greater throughput with enhanced sensitivity for compounds that exhibit temperature dependent inhibition.
2021
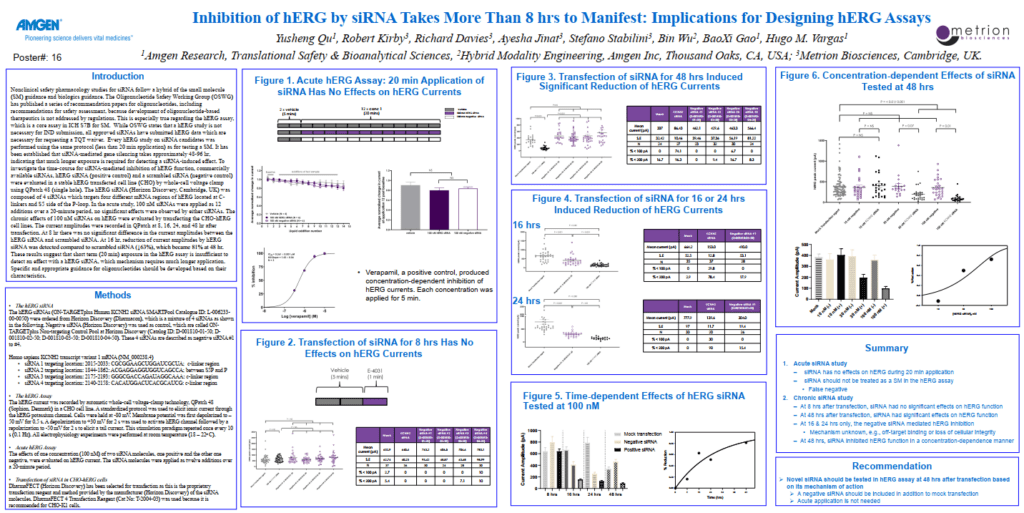
Inhibition of hERG by siRNA Takes More Than 8 hrs to Manifest: Implications for Designing hERG Assays
Safety Pharmacology Society Annual Meeting, Virtual, 2021
Introduction
Nonclinical safety pharmacology studies for siRNA follow a hybrid of the small molecule
(SM) guidance and biologics guidance. The Oligonucleotide Safety Working Group (OSWG)
has published a series of recommendation papers for oligonucleotides, including
recommendations for safety assessment, because development of oligonucleotide-based
therapeutics is not addressed by regulations. This is especially true regarding the hERG assay,
which is a core assay in ICH S7B for SM. While OSWG states that a hERG study is not
necessary for IND submission, all approved siRNAs have submitted hERG data which are
necessary for requesting a TQT waiver.
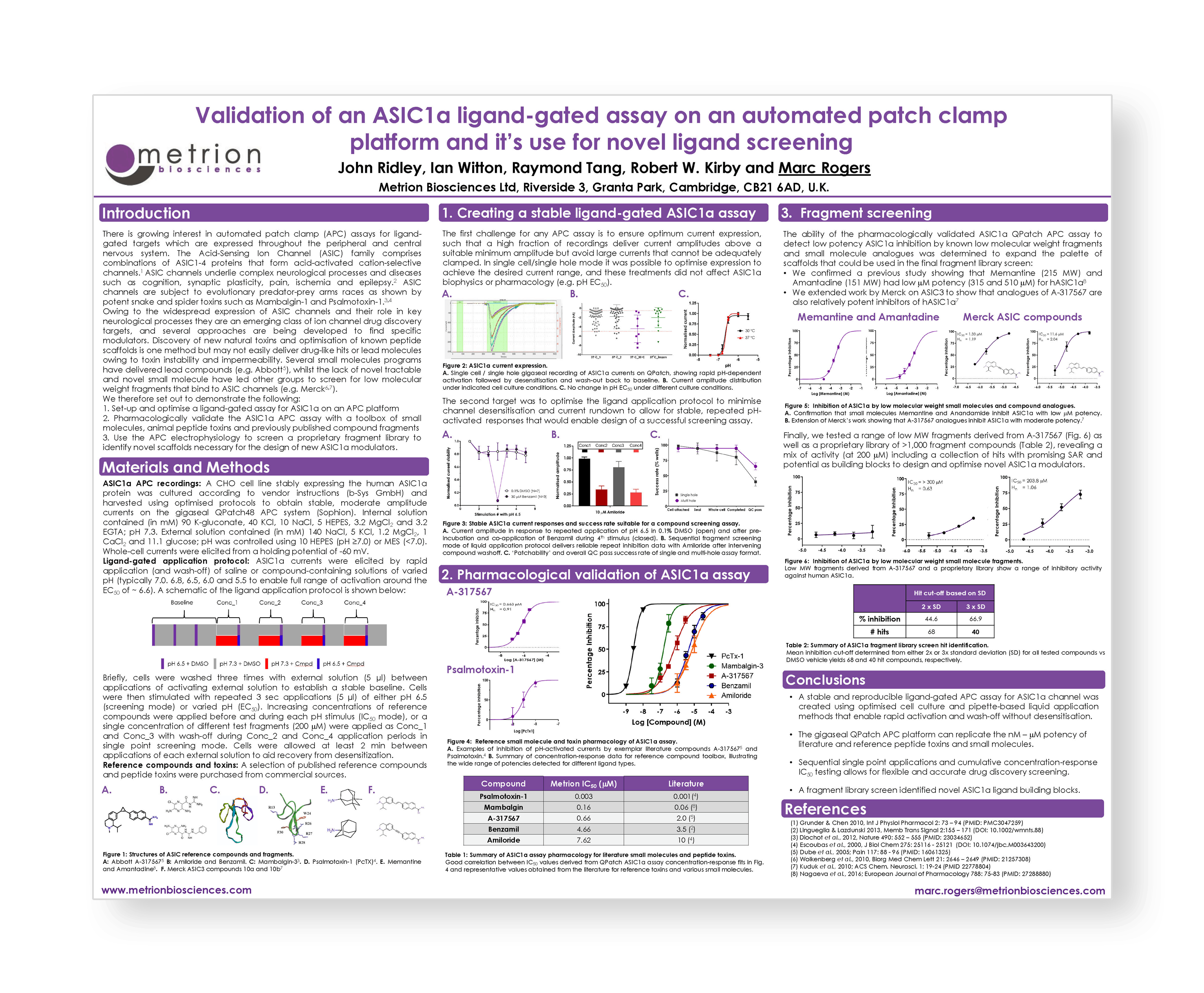
Validation of an ASIC1a ligand-gated assay on an automated patch-clamp platform and it’s use for novel ligand screening
65th Biophysical Society Annual Meeting, Virtual, 2021
Introduction
There is growing interest in automated patch-clamp (APC) assays for ligand-gated targets which are expressed throughout the peripheral and central nervous system. The Acid-Sensing Ion Channel (ASIC) family comprises combinations of ASIC1-4 proteins that form acid-activated cation-selective channels. ASIC channels underlie complex neurological processes and diseases such as cognition, synaptic plasticity, pain, ischemia and epilepsy. ASIC channels are subject to evolutionary predator-prey arms races as shown by potent snake and spider toxins such as Mambalgin-1 and Psalmotoxin-1.
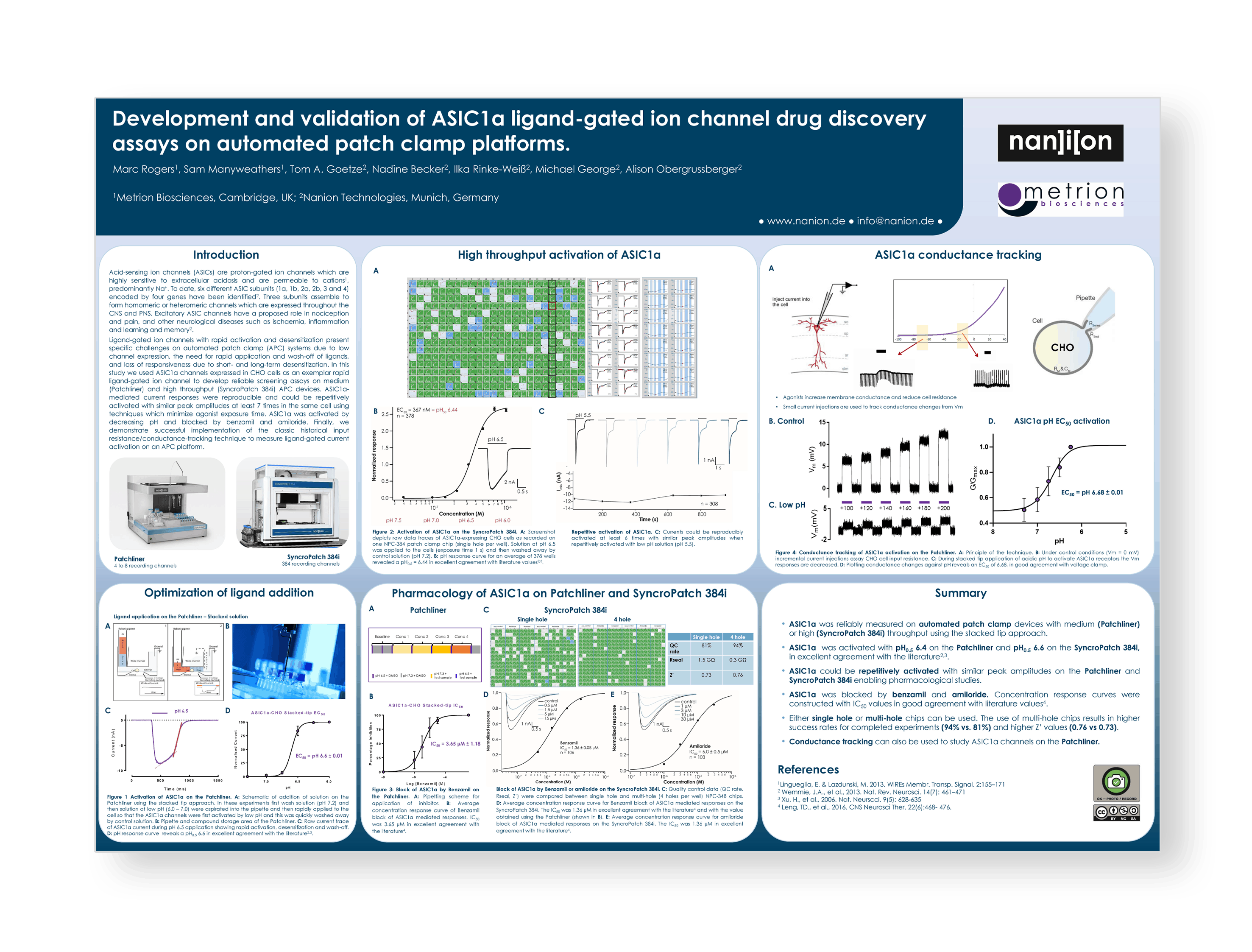
Development and validation of ASIC1a ligand-gated ion channel drug discovery assays on automated patch-clamp platforms (Collaboration with Nanion Technologies)
65th Biophysical Society Annual Meeting, Virtual, 2021
Introduction
Acid-sensing ion channels (ASICs) are proton-gated ion channels which are highly sensitive to extracellular acidosis and are permeable to cations1, predominantly Na+. To date, six different ASIC subunits (1a, 1b, 2a, 2b, 3 and 4) encoded by four genes have been identified. Three subunits assemble to form homomeric or heteromeric channels which are expressed throughout the CNS and PNS. Excitatory ASIC channels have a proposed role in nociception and pain, and other neurological diseases such as ischaemia, inflammation and learning and memory.
2020
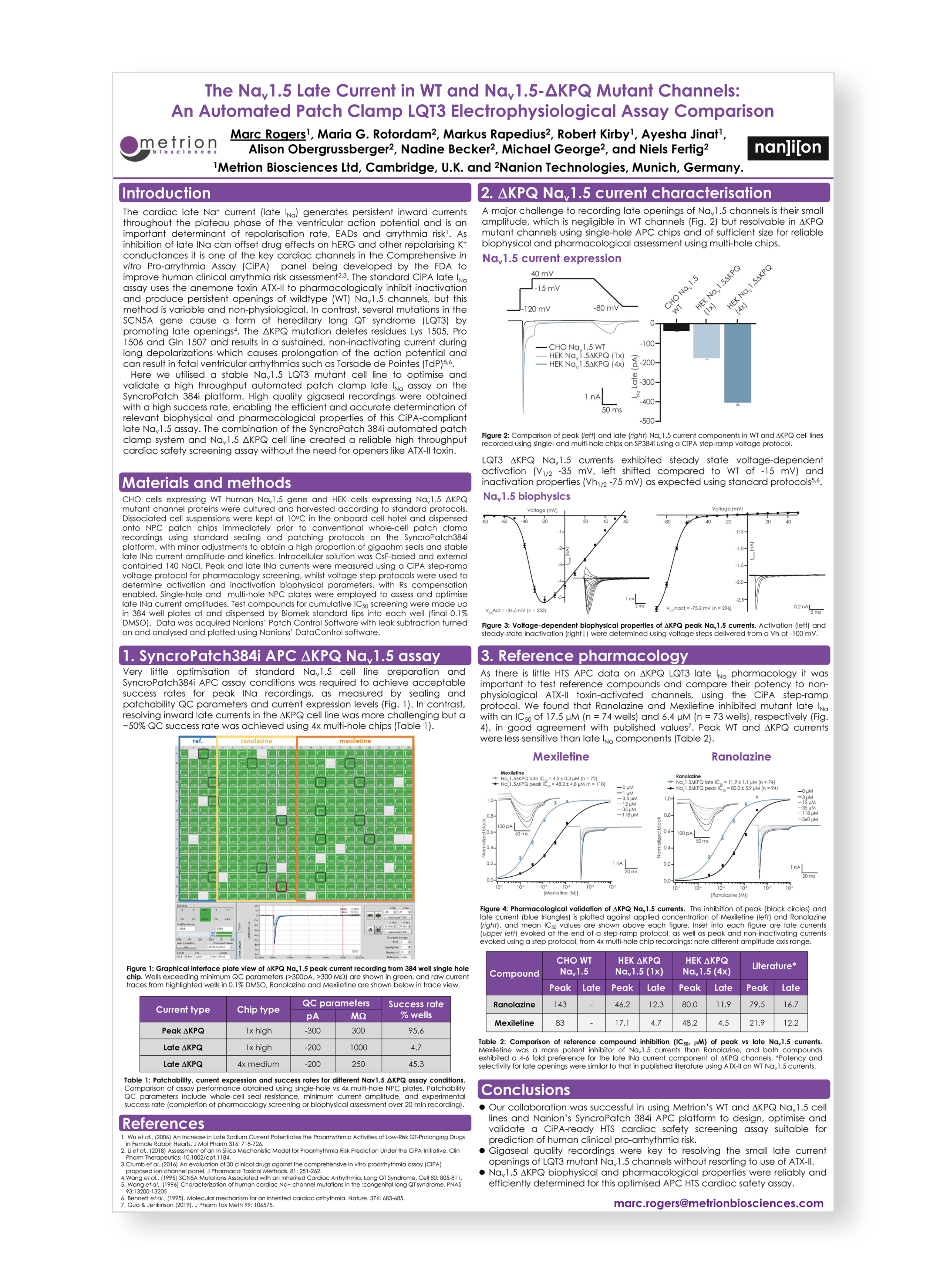
Safety Pharmacology Society (SPS) Annual meeting, Virtual, 2020
Introduction
The cardiac late Na+ current (late INa) generates persistent inward currents throughout the plateau phase of the ventricular action potential and is an important determinant of repolarisation rate, EADs and arrythmia risk. As inhibition of late INa can offset drug effects on hERG and other repolarising K+ conductances it is one of the key cardiac channels in the Comprehensive in vitro Proarrythmia Assay CiPA panel being developed by the FDA to improve human clinical arrythmia risk assessment.
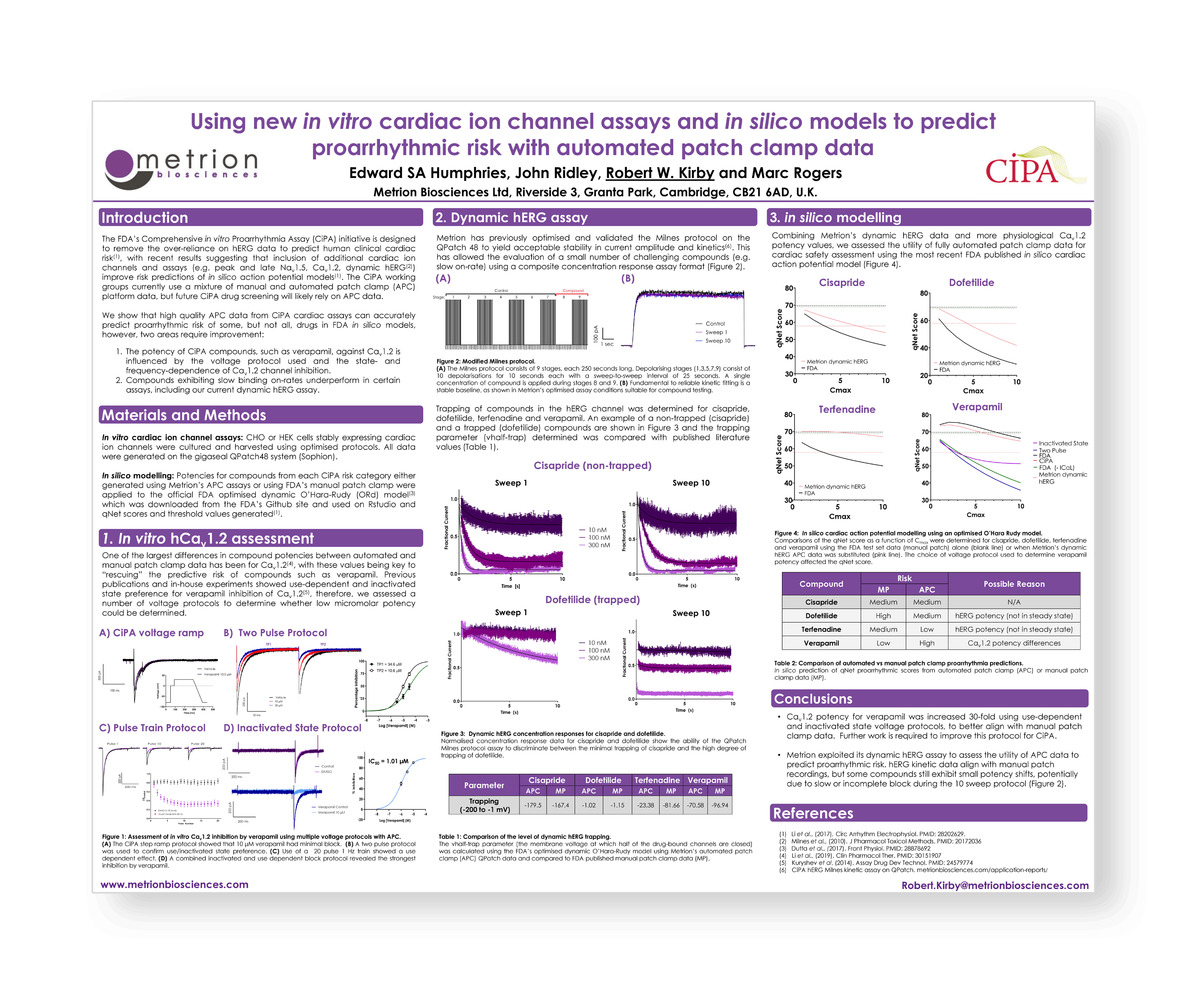
Using new in vitro cardiac ion channel assays and in silico models to predict proarrhythmia risk with automated patch-clamp
Biophysical Society Annual Meeting, San Diego, 2020
Introduction
The FDA’s Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative is designed to remove the over-reliance on hERG data to predict human clinical cardiac risk, with recent results suggesting that inclusion of additional cardiac ion channels and assays (e.g. peak and late Nav1.5, Cav1.2, dynamic hERG) improve risk predictions of in silico action potential models. The CiPA working groups currently use a mixture of manual and automated patch-clamp (APC) platform data, but future CiPA drug screening will likely rely on APC data.
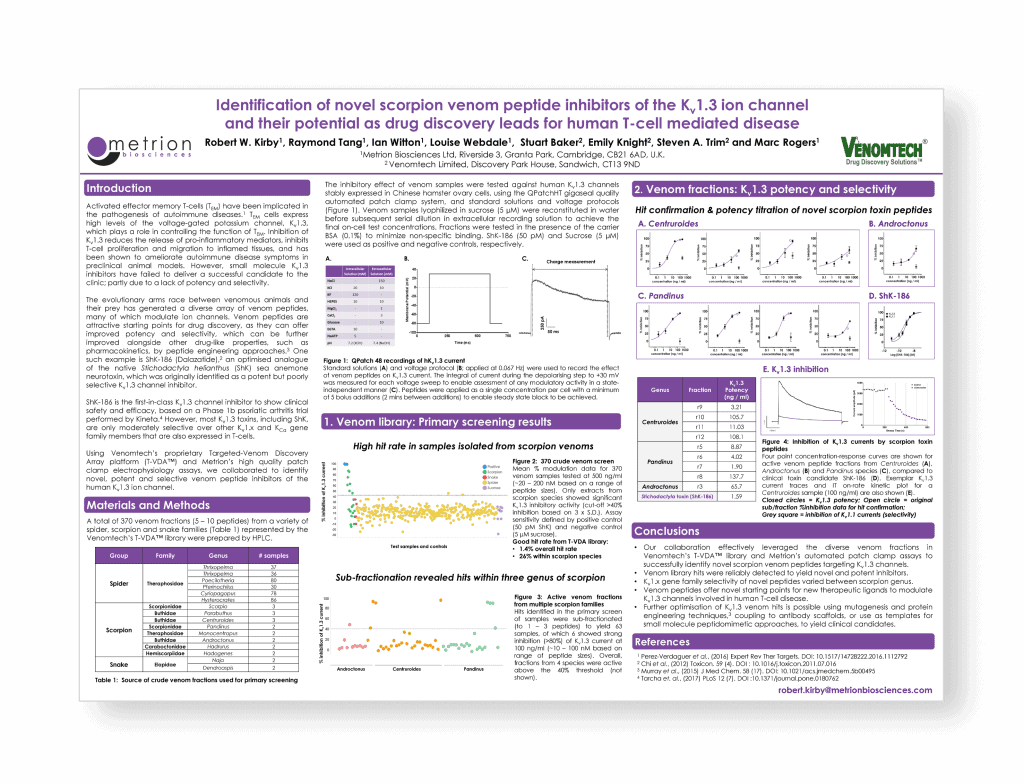
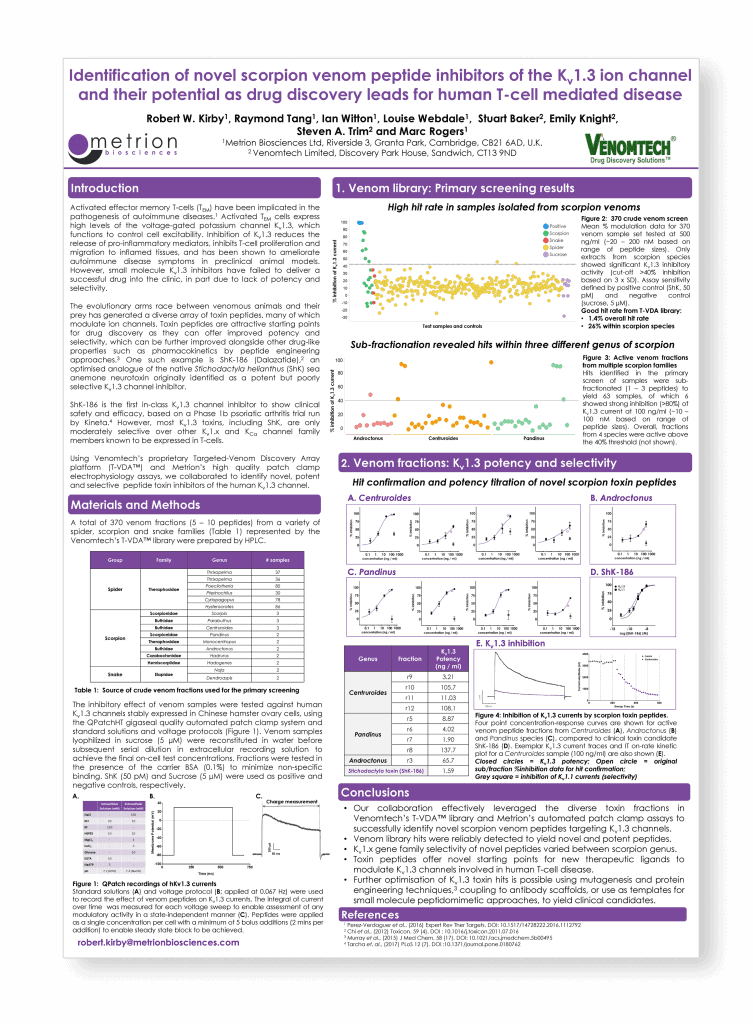
Identification of novel scorpion venom peptide inhibitors of the Kv1.3 ion channel and their potential as drug discovery leads for human T-cell mediated disease
RSC Ion Channels Symposium, Cambridge, 2020
Introduction
Activated effector memory T-cells (TEM) have been implicated in the pathogenesis of autoimmune diseases.1 TEM cells express high levels of the voltage-gated potassium channel, Kv1.3, which plays a role in controlling the function of TEM. Inhibition of Kv1.3 reduces the release of pro-inflammatory mediators, inhibits T-cell proliferation and migration to inflamed tissues, and has been shown to ameliorate autoimmune disease symptoms in preclinical animal models. However, small molecule Kv1.3 inhibitors have failed to deliver a successful candidate to the clinic; partly due to a lack of potency and selectivity.
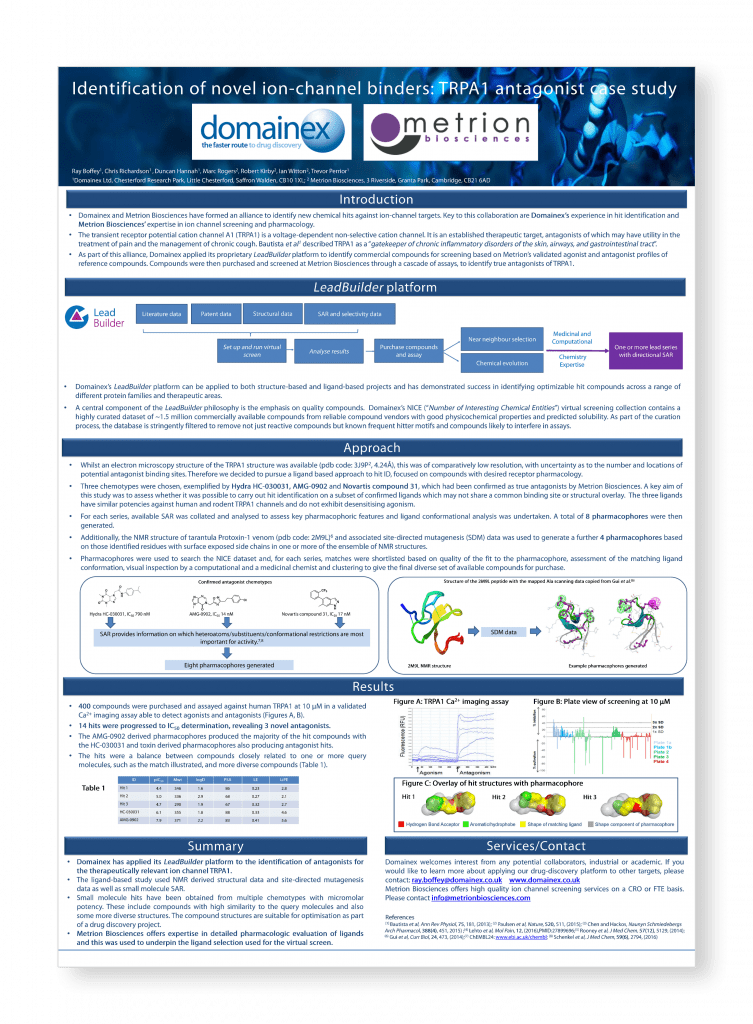
Identification of novel ion channel binders: TRPA1 antagonist case study (Collaboration with Domainex)
RSC Ion Channels Symposium, Cambridge, 2020
Introduction
Domainex and Metrion Biosciences have formed an alliance to identify new chemical hits against ion-channel targets. Key to this collaboration are Domainex’s experience in hit identification and Metrion Bioscience’s expertise in ion channel screening and pharmacology.
The transient receptor potential cation channel A1 (TRPA1) is a voltage-dependent non-selective cation channel. It is an established therapeutic target, antagonists of which may have utility in the treatment of pain and the management of chronic cough. Bautista et al¹ described TRPA1 as a “gatekeeper of chronic inflammatory disorders of the skin, airways, and gastrointestinal tract”.
2019
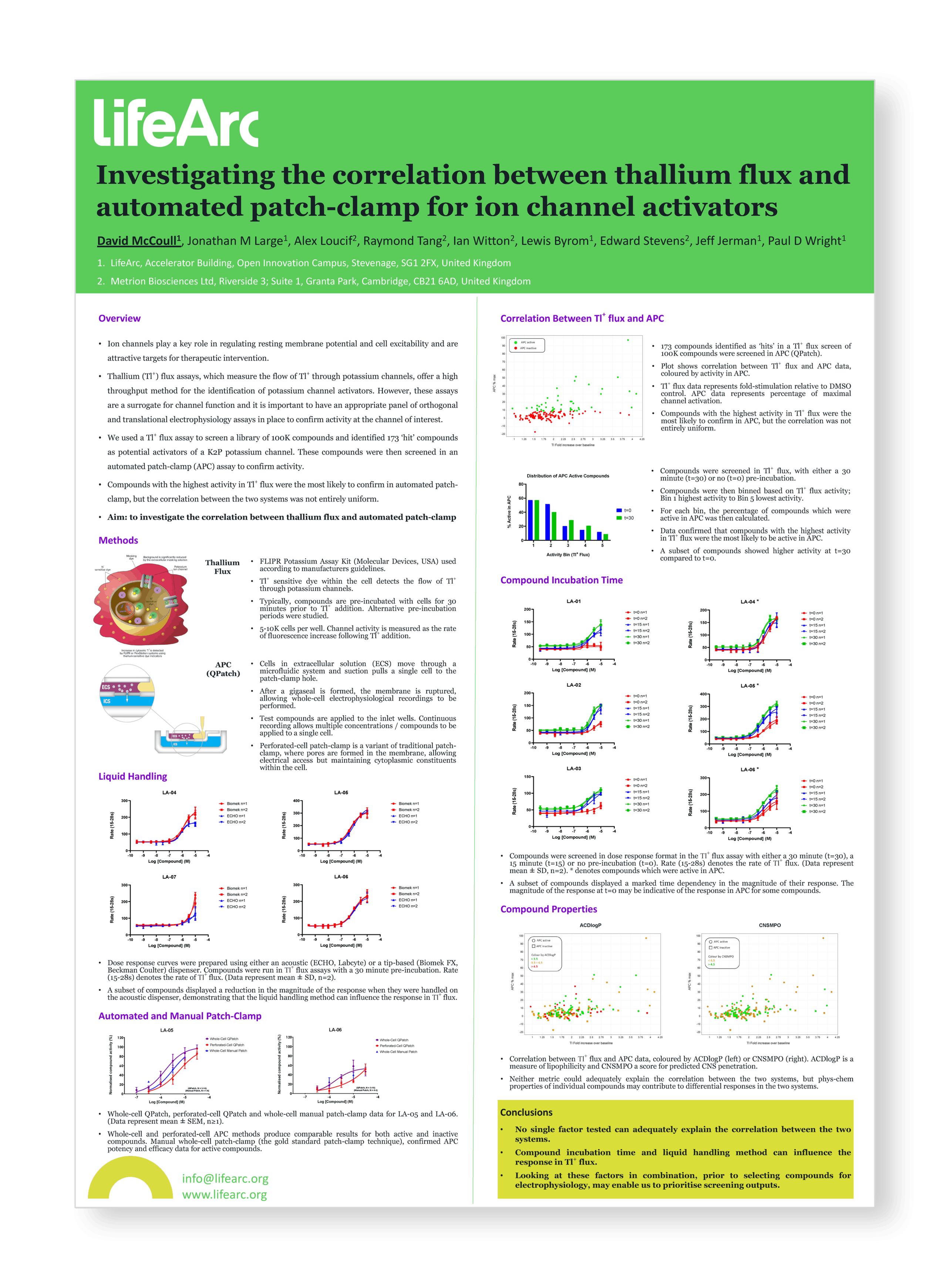
Investigating the correlation between thallium flux and automated patch-clamp for ion channel activators
ELRIG Drug Discovery Annual Meeting, Liverpool, 2019
Introduction
Ion channels play a key role in regulating resting membrane potential and cell excitability and are attractive targets for therapeutic intervention.
Thallium (Tl+) flux assays, which measure the flow of Tl+ through potassium channels, offer a high throughput method for the identification of potassium channel activators. However, these assays are a surrogate for channel function and it is important to have an appropriate panel of orthogonal and translational electrophysiology assays in place to confirm activity at the channel of interest.
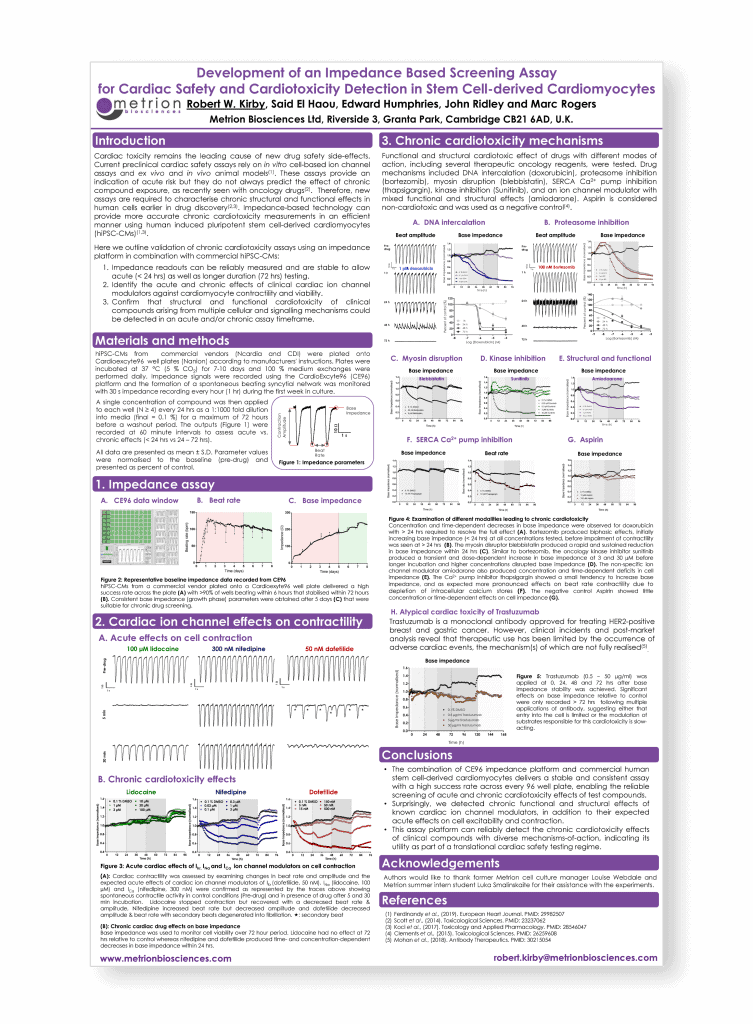
Development of an impedance based screening assay for cardiac safety and cardiotoxicity detection in stem cell derived cardiomyocytes
Safety Pharmacology Society Annual Meeting, Barcelona, 2019
Introduction
Cardiac toxicity remains the leading cause of new drug safety side-effects. Current preclinical cardiac safety assays rely on in vitro cell-based ion channel assays and ex vivo and in vivo animal models. These assays provide an indication of acute risk but they do not always predict the effect of chronic compound exposure, as recently seen with oncology drugs. Therefore, new assays are required to characterise chronic structural and functional effects in human cells earlier in drug discovery. Impedance-based technology can provide more accurate chronic cardiotoxicity measurements in an efficient manner using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).
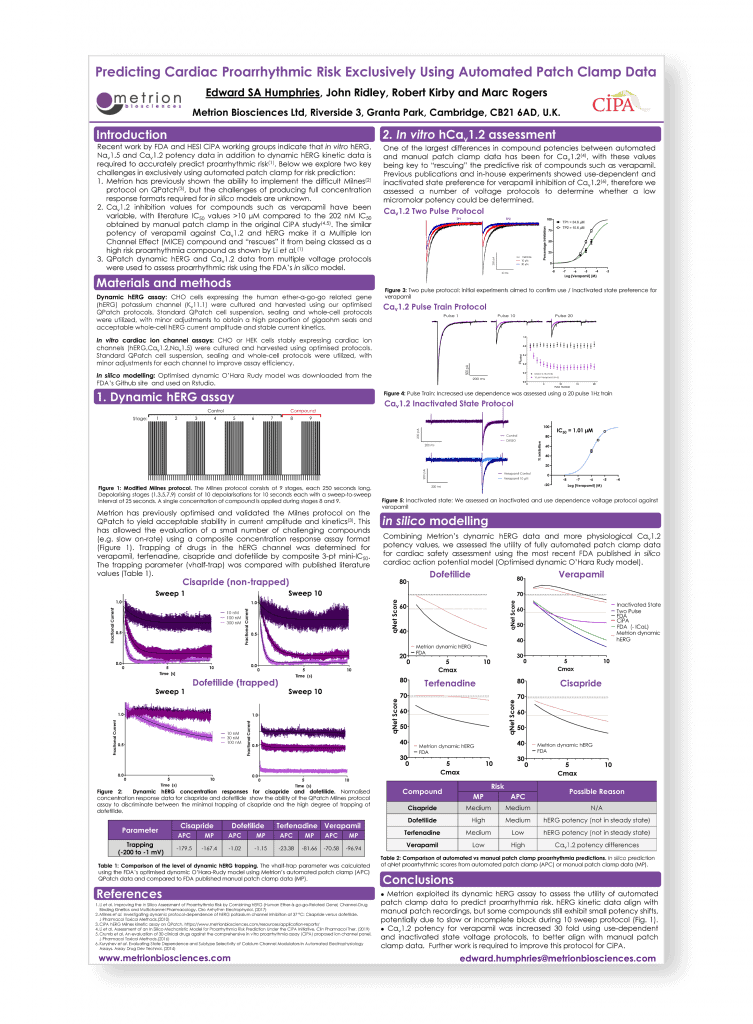
Predicting cardiac proarrhythmic risk exclusively using automated patch-clamp data
Safety Pharmacology Society Annual Meeting, Barcelona, 2019
Introduction
Recent work by FDA and HESI CiPA working groups indicate that in vitro hERG, Nav1.5 and Cav1.2 potency data in addition to dynamic hERG kinetic data is required to accurately predict proarrhythmic risk. Below we explore two key challenges in exclusively using automated patch-clamp for risk prediction:
- Metrion has previously shown the ability to implement the difficult Milnes protocol on QPatch, but the challenges of producing full concentration response formats required for in silico models are unknown.
- Estimating accurate potencies for Cav1.2 inhibitors such as verapamil that match manual patch-clamp values and allow prediction of Multiple Ion Channel Effect (MICE) profiles with reduced proarrhythmia risk.
Identification of novel ion-channel binders: TRPA1 antagonist case study (Collaboration with Domainex)
World Preclinical Congress (WPC), Boston, 2019
Introduction
Domainex and Metrion Biosciences have formed an alliance to identify new chemical hits against ion-channel targets. Key to this collaboration are Domainex’s experience in hit identification and Metrion Bioscience’s expertise in ion channel screening and pharmacology.
The transient receptor potential cation channel A1 (TRPA1) is a voltage-dependent non-selective cation channel. It is an established therapeutic target, antagonists of which may have utility in the treatment of pain and the management of chronic cough. Bautista et al¹ described TRPA1 as a “gatekeeper of chronic inflammatory disorders of the skin, airways, and gastrointestinal tract”.
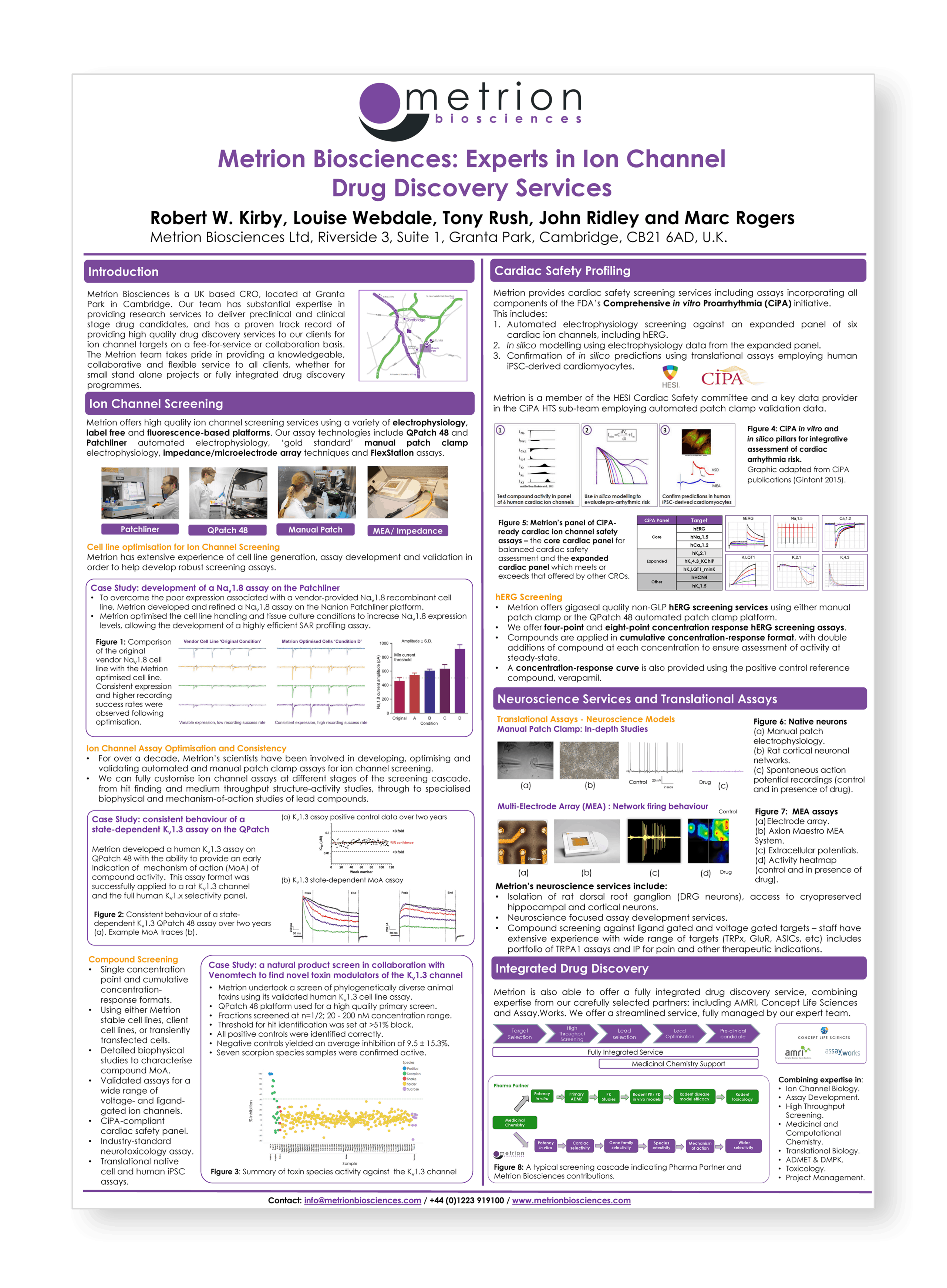
Metrion Biosciences: High quality ion channel drug discovery service provider
Milner Therapeutics Symposium, Cambridge, 2019
Introduction
Metrion Biosciences is a UK based CRO, located at Granta Park in Cambridge. Our team has substantial expertise in providing research services to deliver preclinical and clinical stage drug candidates, and has a proven track record of providing high quality drug discovery services to our customers for ion channel targets on a fee-for-service or collaboration basis. The Metrion team takes pride in providing a knowledgeable, collaborative and flexible service to all customers, whether for small stand alone projects or fully integrated drug discovery programmes.
The development of a set of novel small molecule inhibitors of the Kv1.3 ion channel
Biotech Outsourcing Strategy (BOS) Basel, 2019
Introduction
Ion channels represent 15 – 20% of historic drug approvals and recent drug discovery projects. Many ion channel families (Nav, Cav, TRPx and GABA) are validated as therapeutic targets based on human genetics, animal models and selective pharmacology. However, ion channels are challenging targets requiring expert target class knowledge and specialised screening technology such as automated patch-clamp (APC) electrophysiology.
2018
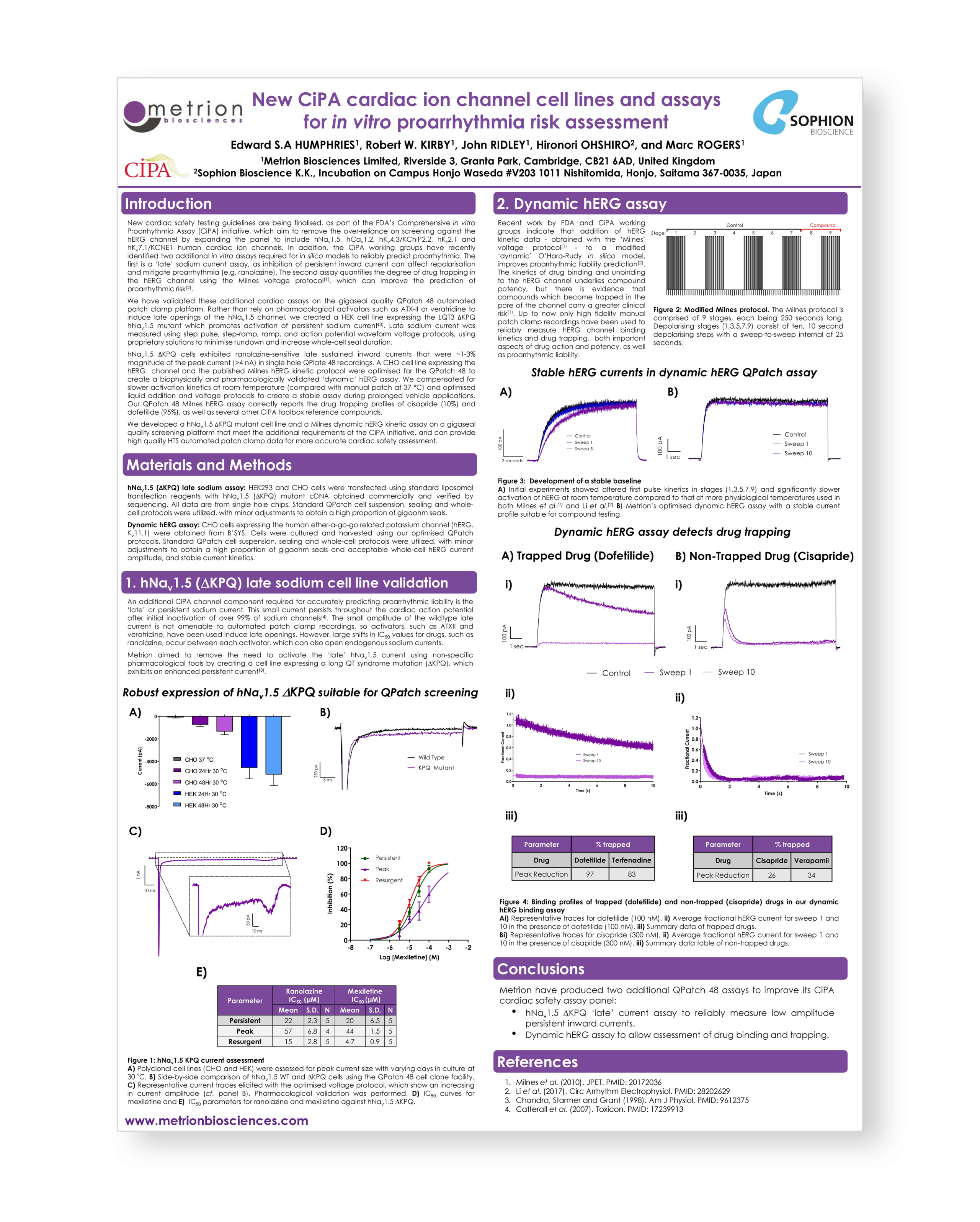
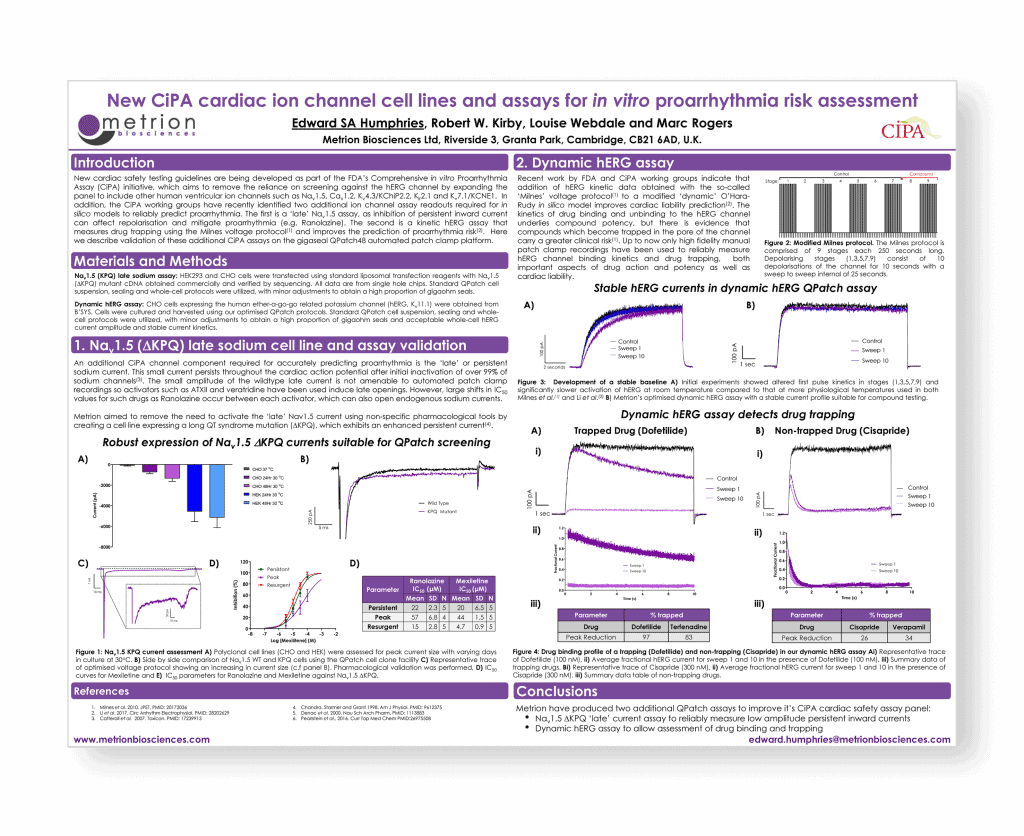
New CiPA cardiac ion channel cell lines and assays for in vitro proarrhythmia risk assessment
Safety Pharmacology Society (SPS) Annual meeting, Washington DC, 2018
Introduction
New cardiac safety testing guidelines are being finalised, as part of the FDA’s Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, which aim to remove the over-reliance on screening against the hERG channel by expanding the panel to include hNav1.5, hCav1.2, hKv4.3/KChiP2.2, hKir2.1 and hKv7.1/KCNE1 human cardiac ion channels. In addition, the CiPA working groups have recently identified two additional in vitro assays required for in silico models to reliably predict proarrhythmia. The first is a ‘late’ sodium current assay, as inhibition of persistent inward current can affect repolarisation and mitigate proarrhythmia (e.g. ranolazine). The second assay quantifies the degree of drug trapping in the hERG channel using the Milnes voltage protocol, which can improve the prediction of proarrhythmic risk.
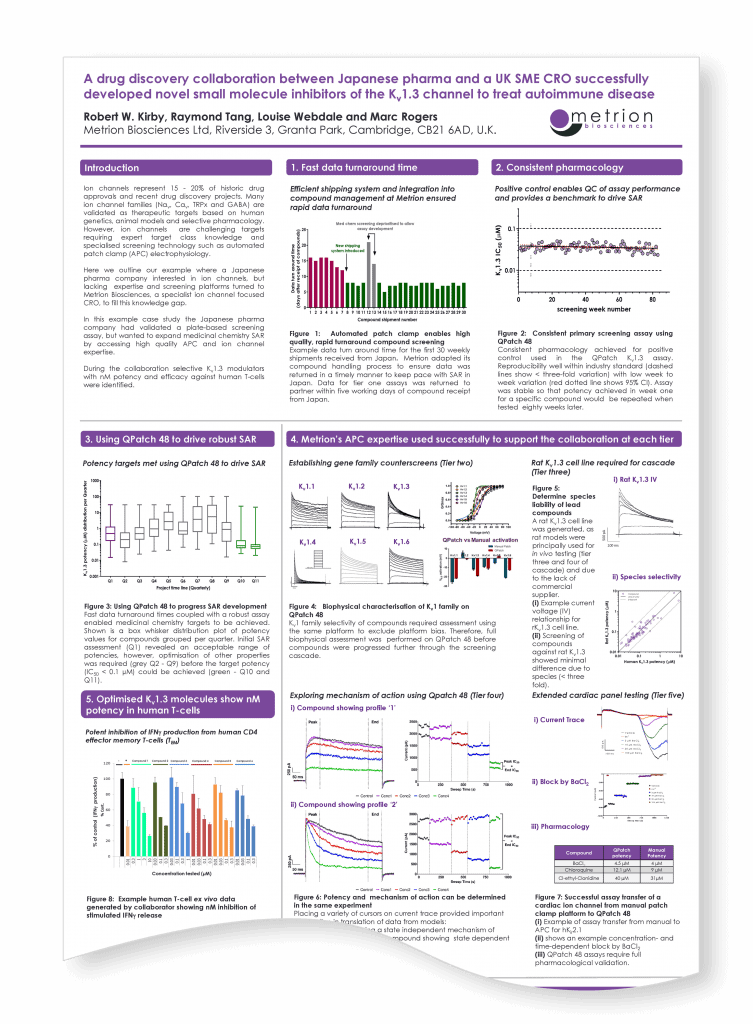
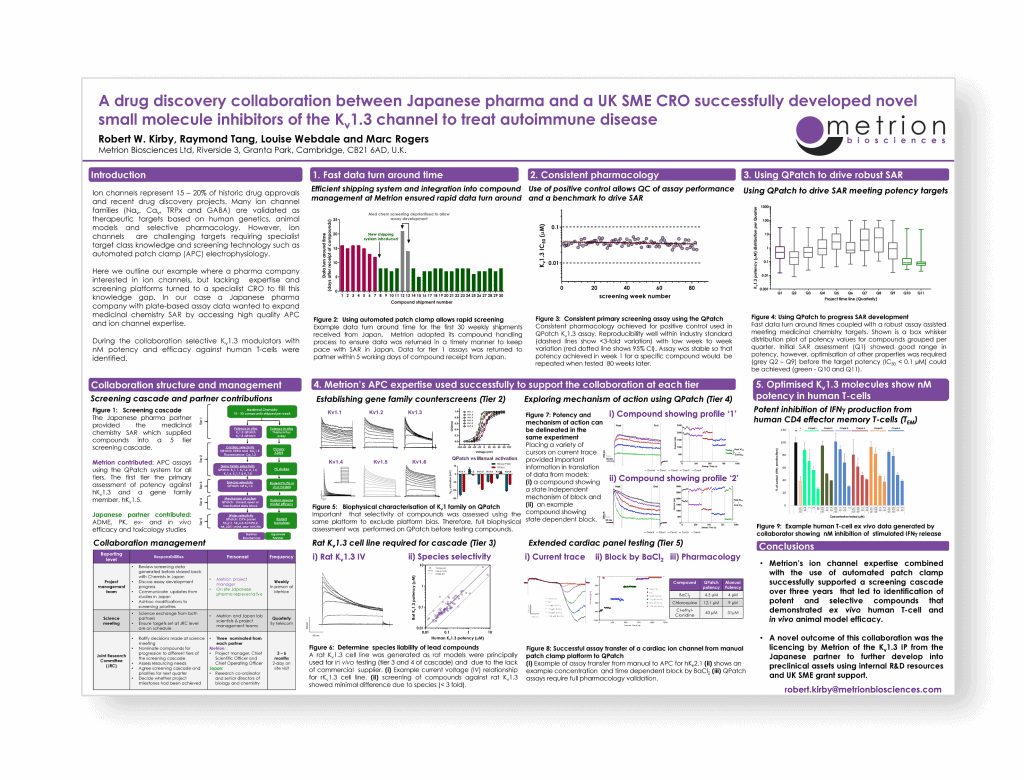
A drug discovery collaboration between Japanese pharma and a UK SME CRO successfully developed novel small molecule inhibitors of the Kv1.3 channel to treat autoimmune disease
The Best of Both Worlds: Innovation, Collaboration and Synergy between CROs and their Customer Partners, Stevenage, 2018
Introduction
Here we outline our example where a pharma company interested in ion channels, but lacking expertise and screening platforms turned to a specialist CRO to fill this knowledge gap. In our case a Japanese pharma company with plate-based assay data wanted to expand medicinal chemistry SAR by accessing high quality APC and ion channel expertise.
During the collaboration selective Kv1.3 modulators with nM potency and efficacy against human T-cells were identified.
Identification of novel scorpion venom peptide inhibitors of the Kv1.3 ion channel and their potential as drug discovery leads for human T-cell mediated disease
ELRIG Drug Discovery, London, 2018
Introduction
Activated effector memory T-cells (TEM) have been implicated in the pathogenesis of autoimmune diseases.1 Activated TEM cells express high levels of the voltage-gated potassium channel Kv1.3, which functions to control cell excitability. Inhibition of Kv1.3 reduces the release of pro-inflammatory mediators, inhibits T-cell proliferation and migration to inflamed tissues, and has been shown to ameliorate autoimmune disease symptoms in preclinical animal models. However, small molecule Kv1.3 inhibitors have failed to deliver a successful drug into the clinic, in part due to lack of potency and selectivity.
New CiPA cardiac ion channel cell lines and assays for in vitro proarrhythmia risk assessment
Safety Pharmacology Society meeting, Washington DC, USA 2018
Introduction
New cardiac safety testing guidelines are being developed as part of the FDA’s Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, which aims to remove the reliance on screening against the hERG channel by expanding the panel to include other human ventricular ion channels such as Nav1.5, Cav1.2, Kv4.3/KChiP2.2, Kir2.1 and Kv7.1/KCNE1. In addition, the CiPA working groups have recently identified two additional ion channel assay readouts required for in silico models to reliably predict proarrhythmia. The first is a ‘late’ Nav1.5 assay, as inhibition of persistent inward current can affect repolarisation and mitigate proarrhythmia (e.g. Ranolazine). The second is a kinetic hERG assay that measures drug trapping using the Milnes voltage protocol(1) and improves the prediction of proarrhythmia risk. Here we describe validation of these additional CiPA assays on the gigaseal QPatch48 automated patch-clamp platform.
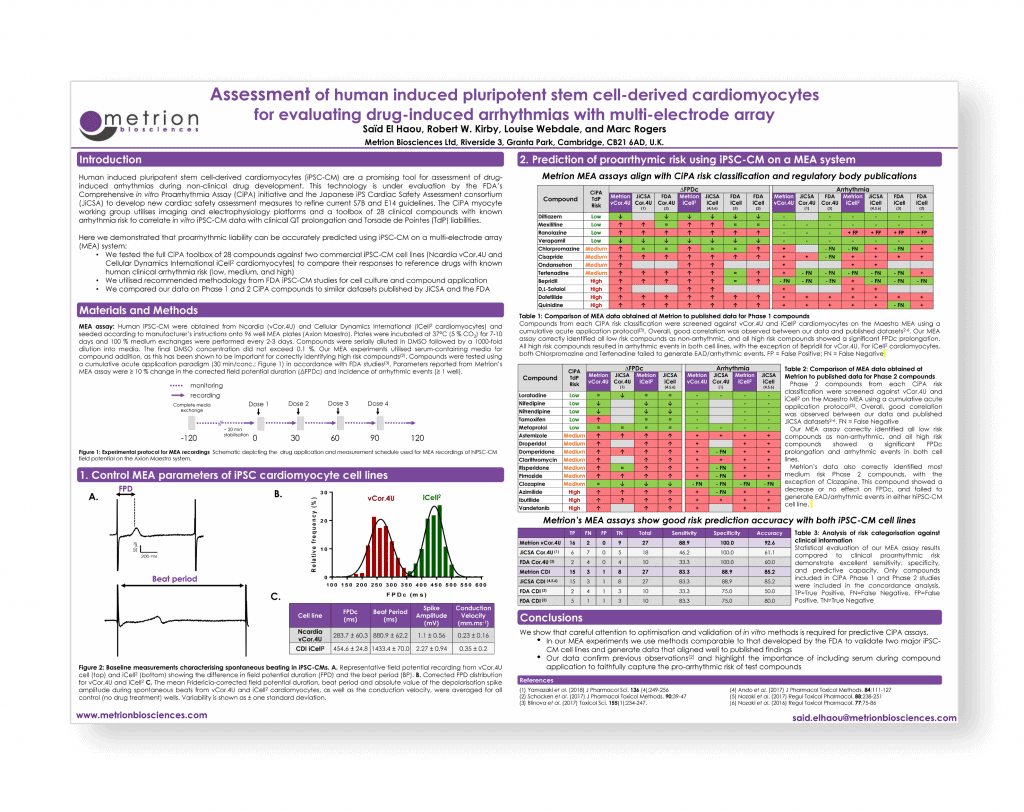
Assessment of human induced pluripotent stem cell-derived cardiomyocytes for evaluating drug-induced arrhythmias with multi-electrode array
Safety Pharmacology Society (SPS) Annual meeting, Washington DC, 2018
Introduction
Human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) are a promising tool for assessment of drug-induced arrhythmias during non-clinical drug development. This technology is under evaluation by the FDA’s Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative and the Japanese iPS Cardiac Safety Assessment consortium (JiCSA) to develop new cardiac safety assessment measures to refine current S7B and E14 guidelines. The CiPA myocyte working group utilises imaging and electrophysiology platforms and a toolbox of 28 clinical compounds with known arrhythmia risk to correlate in vitro iPSC-CM data with clinical QT prolongation and Torsade de Pointes (TdP) liabilities.
Functional characterisation of human iPSC-derived atrial cardiomyocytes
SelectBio Stem Cells and Antibodies in Drug Discovery, Cambridge, May 2018
Introduction
Atrial fibrillation (AF) is the most common arrhythmia observed in the clinic, considerable effort has been made to identify the cellular mechanisms of AF and develop new safe and effective antiarrhythmic drugs(1). However, preclinical studies using non-cardiac cells and non-human animal models may not replicate the physiology of human atrial cardiomyocytes or predict patient efficacy and safety.
Here we outline our results from studies to validate human induced pluripotent stem cell-derived atrial cardiomyocytes (hiPSC-ACMs) generated by Axol Bioscience.
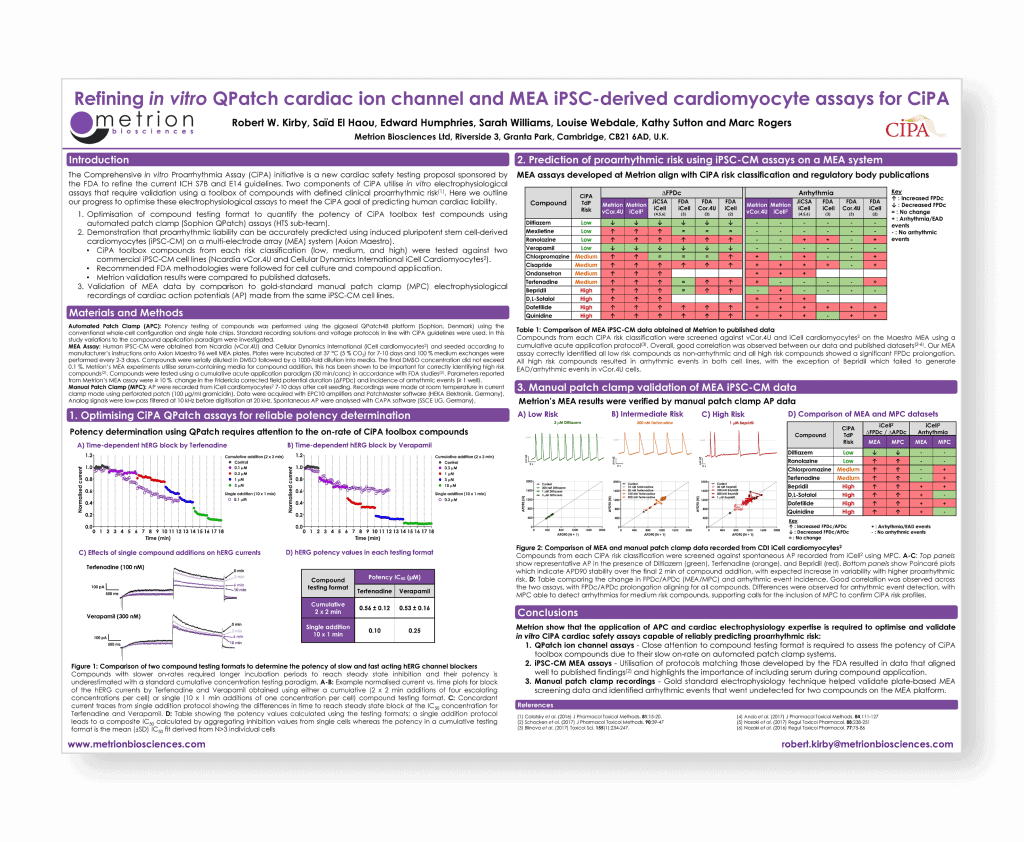
Refining in vitro QPatch cardiac ion channel QPatch and MEA iPSC cardiomyocyte assays for CiPA
Society of Toxicology (SoT), San Antonio, 2018
Introduction
The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative is a new cardiac safety testing proposal sponsored by the FDA to refine the current ICH S7B and E14 guidelines. Two components of CiPA utilise in vitro electrophysiological assays that require validation using a toolbox of compounds with defined clinical proarrhythmic risk. Here we outline our progress to optimise these electrophysiological assays to meet the CiPA goal of predicting human cardiac liability.
2017
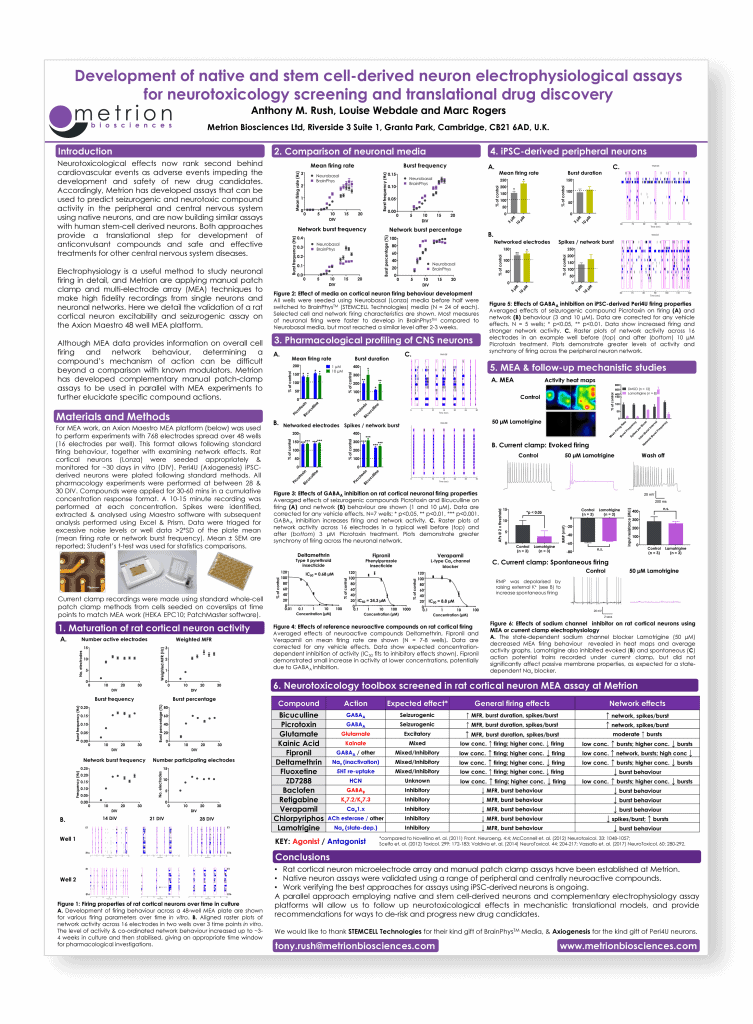
Development of native and stem cell-derived electrophysiological assays for neurotoxicology screening and translational drug discovery
Safety Pharmacology Society (SPS) Annual Meeting, Berlin, 2017 (poster number 142)
Introduction
Neurotoxicological effects now rank second behind cardiovascular events as adverse events impeding the development and safety of new drug candidates. Accordingly, Metrion has developed assays that can be used to predict seizurogenic and neurotoxic compound activity in the peripheral and central nervous system using native neurons, and are now building similar assays with human stem-cell derived neurons. Both approaches provide a translational step for development of anticonvulsant compounds and safe and effective treatments for other central nervous system diseases.
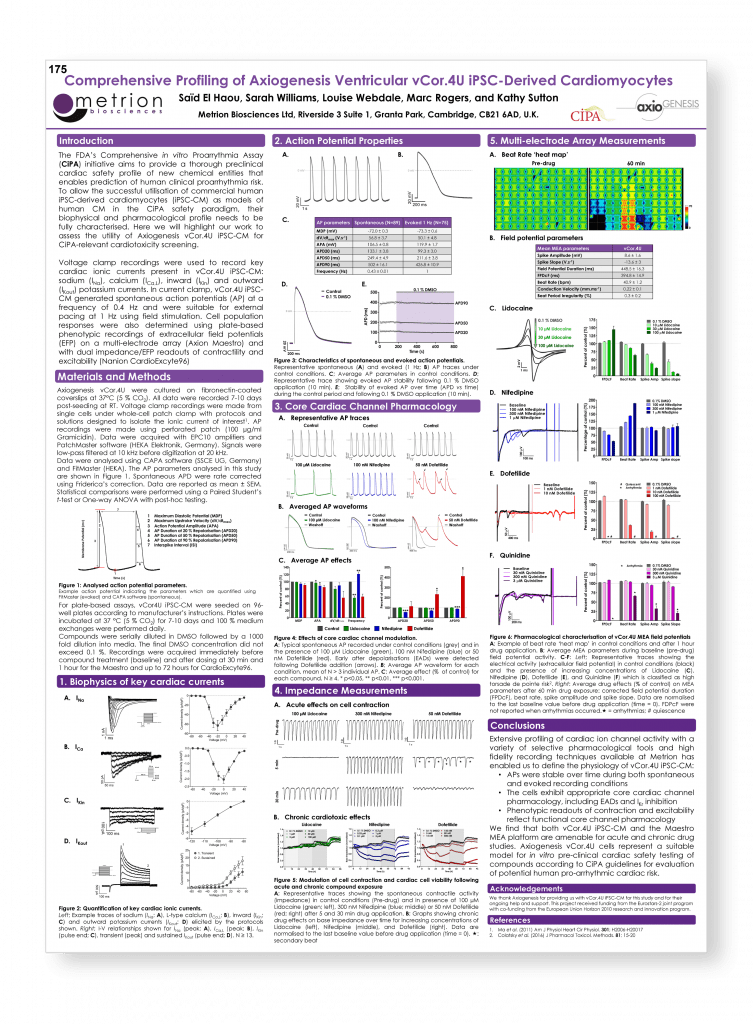
Comprehensive profiling of Axiogenesis ventricular vCor.4U iPSC-derived cardiomyocytes – from electrophysiology to phenotypic assays
Safety Pharmacology Society (SPS) Annual Meeting, Berlin, 2017 (poster number 175)
Introduction
The FDA’s Comprehensive in vitro Proarrythmia Assay (CiPA) initiative aims to provide a thorough preclinical cardiac safety profile of new chemical entities that enables prediction of human clinical proarrhythmia risk. To allow the successful utilisation of commercial human iPSC-derived cardiomyocytes (iPSC-CM) as models of human CM in the CiPA safety paradigm, their biophysical and pharmacological profile needs to be fully characterised. Here we will highlight our work to assess the utility of Axiogenesis vCor.4U iPSC-CM for CiPA-relevant cardiotoxicity screening.
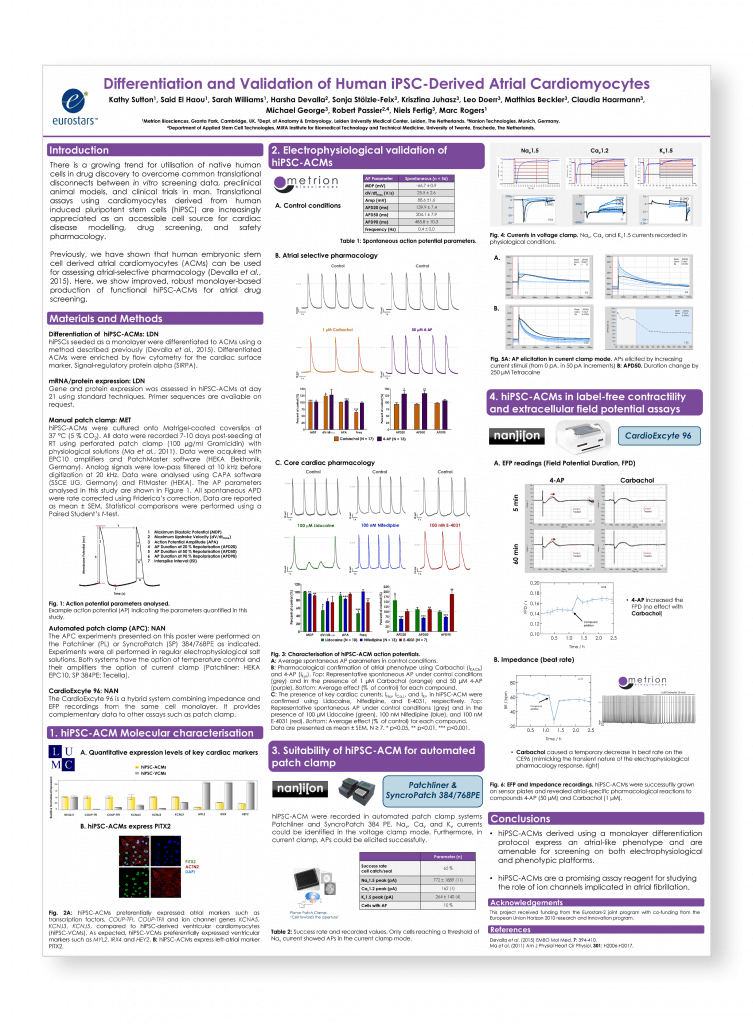
Differentiation and validation of human iPSC-derived atrial cardiomyocytes
Safety Pharmacology Society (SPS) Annual Meeting, Berlin, 2017 (poster number 176)
Introduction
There is a growing trend for utilisation of native human cells in drug discovery to overcome common translational disconnects between in vitro screening data, preclinical animal models, and clinical trials in man. Translational assays using cardiomyocytes derived from human induced pluripotent stem cells (hiPSC) are increasingly appreciated as an accessible cell source for cardiac disease modelling, drug screening, and safety pharmacology.
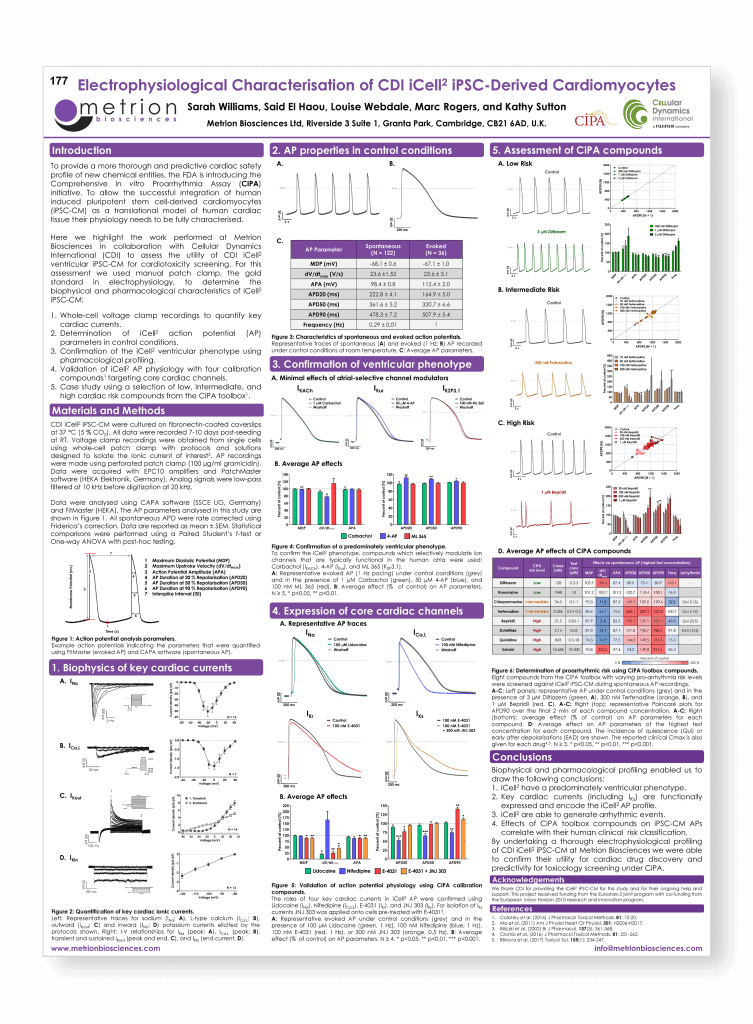
Electrophysiological characterisation of Cellular Dynamics International ventricular iCell2 iPSC-derived cardiomyocytes
Safety Pharmacology Society (SPS) Annual Meeting, Berlin, 2017 (poster number 177)
Introduction
To provide a more thorough and predictive cardiac safety profile of new chemical entities, the FDA is introducing the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative. To allow the successful integration of human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) as a translational model of human cardiac tissue their physiology needs to be fully characterised.
Here we highlight the work performed at Metrion Biosciences in collaboration with Cellular Dynamics International (CDI) to assess the utility of CDI iCell2 ventricular iPSC-CM for cardiotoxicity screening.
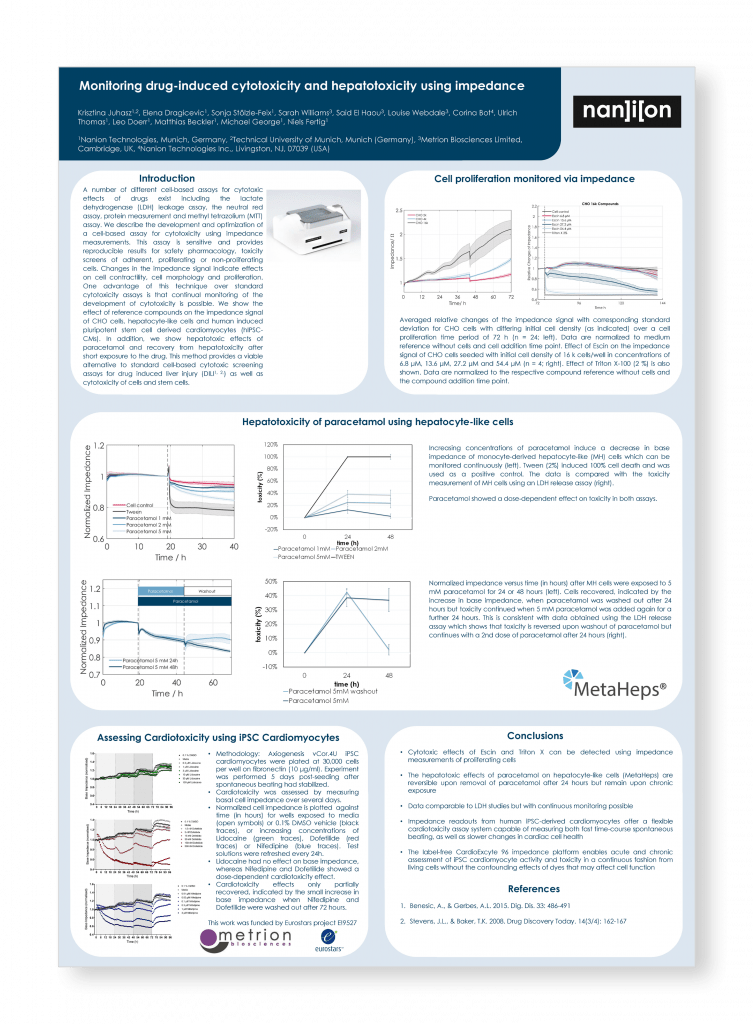
Monitoring drug-induced cytotoxicity and hepatotoxicity using impedance
Advances in Cell Based Screening, Gothenburg, May 2017
Introduction
A number of different cell-based assays for cytotoxic effects of drugs exist including the lactate dehydrogenase (LDH) leakage assay, the neutral red assay, protein measurement and methyl tetrazolium (MTT) assay. We describe the development and optimization of a cell-based assay for cytotoxicity using impedance measurements. This assay is sensitive and provides reproducible results for safety pharmacology, toxicity screens of adherent, proliferating or non-proliferating cells. Changes in the impedance signal indicate effects on cell contractility, cell morphology and proliferation.
2016
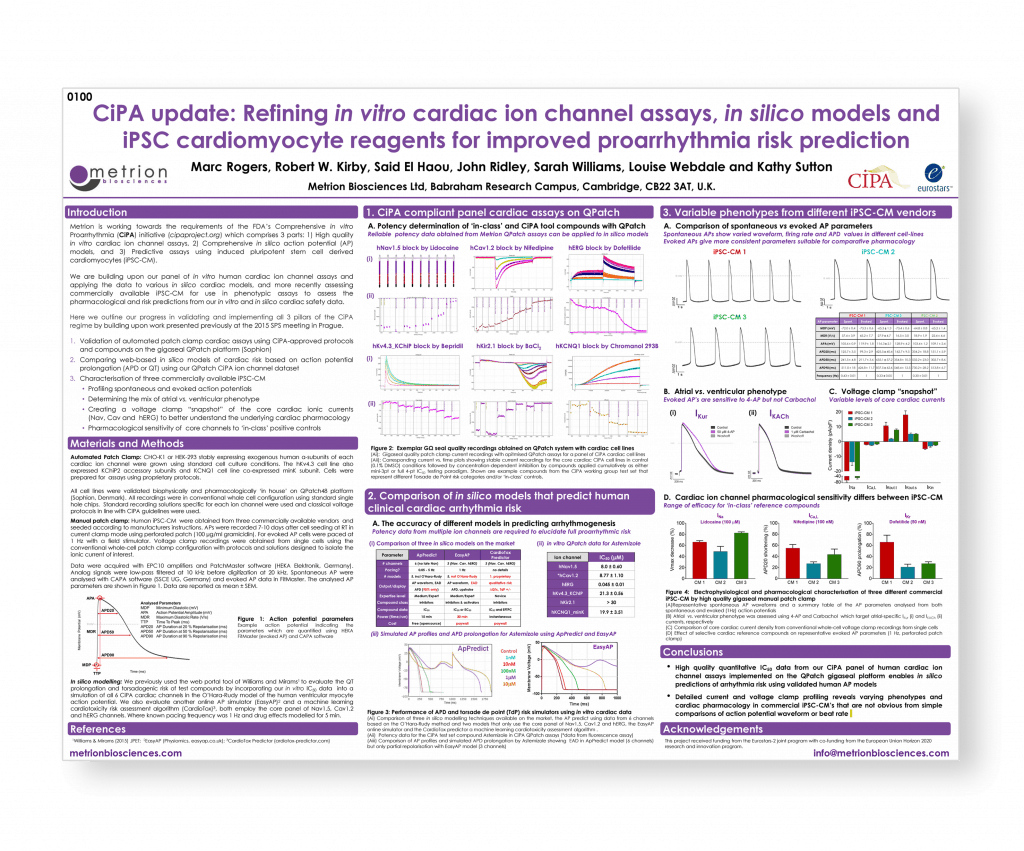
CiPA update: Refining in vitro cardiac ion channel assays, in silico models and iPSC cardiomyocyte reagents for improved proarrhythmia risk prediction
SPS Vancouver September 2016 poster 0100
Introduction
Metrion is working towards the requirements of the FDA’s Comprehensive in vitro Proarrhythmia (CiPA) initiative (cipaproject.org) which comprises 3 parts: 1) High quality in vitro cardiac ion channel assays, 2) Comprehensive in silico action potential (AP) models, and 3) Predictive assays using induced pluripotent stem cell derived cardiomyocytes (iPSC-CM).
We are building upon our panel of in vitro human cardiac ion channel assays and applying the data to various in silico cardiac models, and more recently assessing commercially available iPSC-CM for use in phenotypic assays to assess the pharmacological and risk predictions from our in vitro and in silico cardiac safety data.
Here we outline our progress in validating and implementing all 3 pillars of the CiPA regime by building upon work presented previously at the 2015 SPS meeting in Prague.
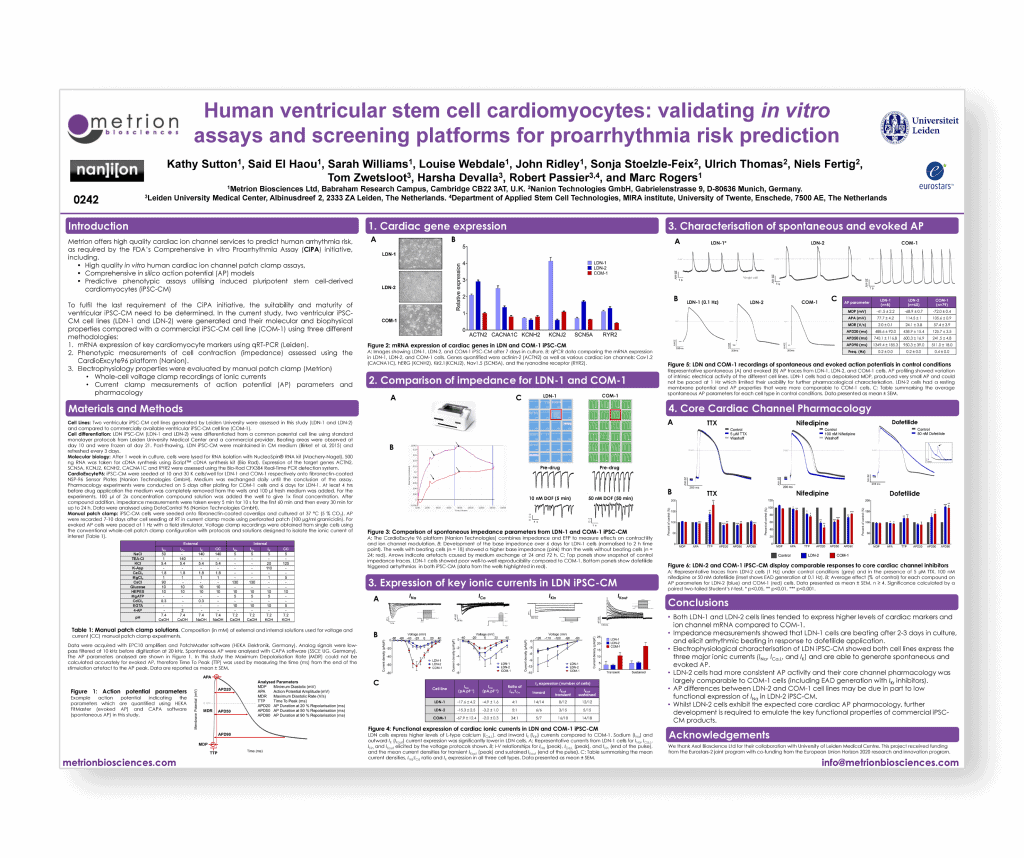
Human ventricular stem cell cardiomyocytes: validating in vitro assays and screening platforms for proarrhythmia risk prediction
SPS Vancouver September 2016 poster 0242
Introduction
To fulfil the last requirement of the CiPA initiative, the suitability and maturity of ventricular iPSC-CM need to be determined. In the current study, two ventricular iPSC- CM cell lines (LDN-1 and LDN-2) were generated and their molecular and biophysical properties compared with a commercial iPSC-CM cell line (COM-1) using three different methodologies.
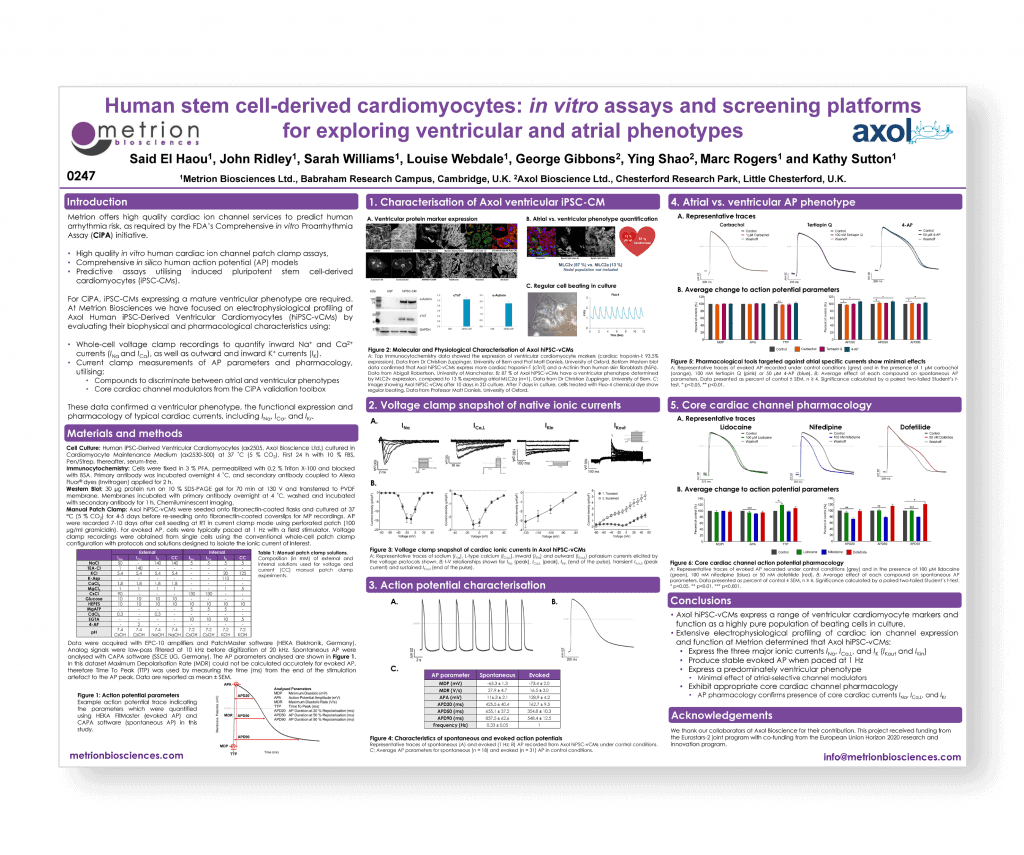
Human stem cell-derived cardiomyocytes: in vitro assays and screening platforms for exploring ventricular and atrial phenotypes
SPS Vancouver September 2016 poster 0247
Introduction
For CiPA, iPSC-CMs expressing a mature ventricular phenotype are required. At Metrion Biosciences we have focused on electrophysiological profiling of Axol Human iPSC-Derived Ventricular Cardiomyocytes (hiPSC-vCMs) by evaluating their biophysical and pharmacological characteristics.
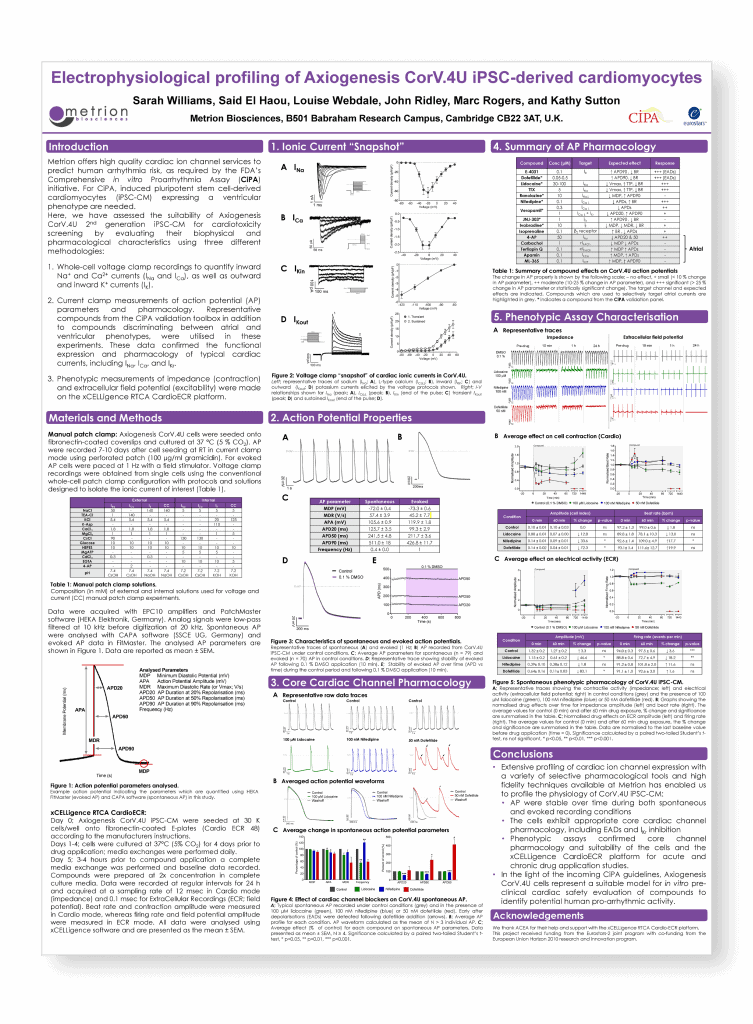
Electrophysiological profiling of Axiogenesis vCor.4U iPSC-derived cardiomyocytes
Axiogenesis Human iPSC Applications Workshop, Cologne, 2016
Introduction
Here, we have assessed the suitability of Axiogenesis CorV.4U 2nd generation iPSC-CM for cardiotoxicity screening by evaluating their biophysical and pharmacological characteristics using three different methodologies:
- Whole-cell voltage clamp recordings to quantify inward Na+ and Ca2+ currents (INa and ICa), as well as outward and inward K+ currents (IK).
- Current clamp measurements of action potential (AP) parameters and pharmacology. Representative compounds from the CiPA validation toolbox in addition to compounds discriminating between atrial and ventricular phenotypes, were utilised in these experiments. These data confirmed the functional expression and pharmacology of typical cardiac currents, including INa, ICa, and IKr.
- Phenotypic measurements of impedance (contraction) and extracellular field potential (excitability) were made on the xCELLigence RTCA CardioECR platform.
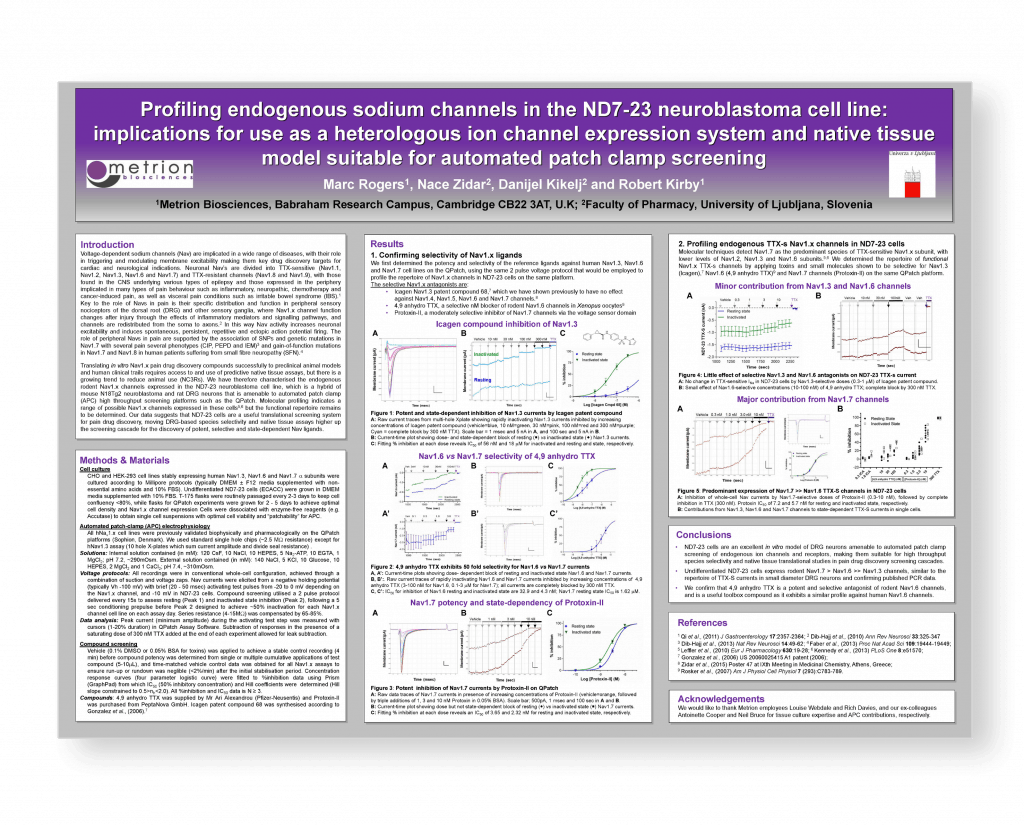
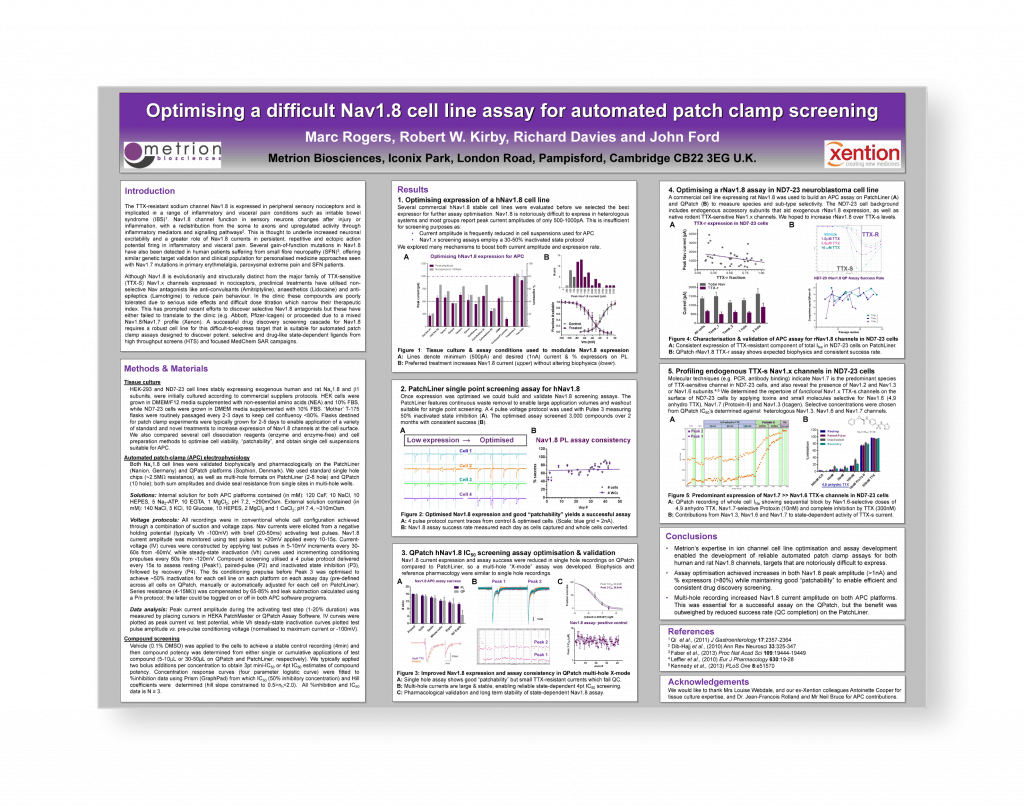
Profiling endogenous sodium channels in the ND7-23 neuroblastoma cell line: implications for use as a heterologous ion channel expression system and native tissue model suitable for automated patch-clamp screening
5th RSC / SCI symposium on Ion Channels as Therapeutic Targets, Cambridge, 2016
Introduction
Voltage-dependent sodium channels (Nav) are implicated in a wide range of diseases, with their role in triggering and modulating membrane excitability making them key drug discovery targets for cardiac and neurological indications. Neuronal Nav’s are divided into TTX-sensitive (Nav1.1, Nav1.2, Nav1.3, Nav1.6 and Nav1.7) and TTX-resistant channels (Nav1.8 and Nav1.9), with those found in the CNS underlying various types of epilepsy and those expressed in the periphery implicated in many types of pain behaviour such as inflammatory, neuropathic, chemotherapy and cancer-induced pain, as well as visceral pain conditions such as irritable bowel syndrome (IBS). Key to the role of Navs in pain is their specific distribution and function in peripheral sensory nociceptors of the dorsal root (DRG) and other sensory ganglia, where Nav1.x channel function changes after injury through the effects of inflammatory mediators and signalling pathways, and channels are redistributed from the soma to axons.2 In this way Nav activity increases neuronal excitability and induces spontaneous, persistent, repetitive and ectopic action potential firing. The role of peripheral Navs in pain are supported by the association of SNPs and genetic mutations in Nav1.7 with several pain several phenotypes (CIP, PEPD and IEM)3 and gain-of-function mutations in Nav1.7 and Nav1.8 in human patients suffering from small fibre neuropathy (SFN).
2015
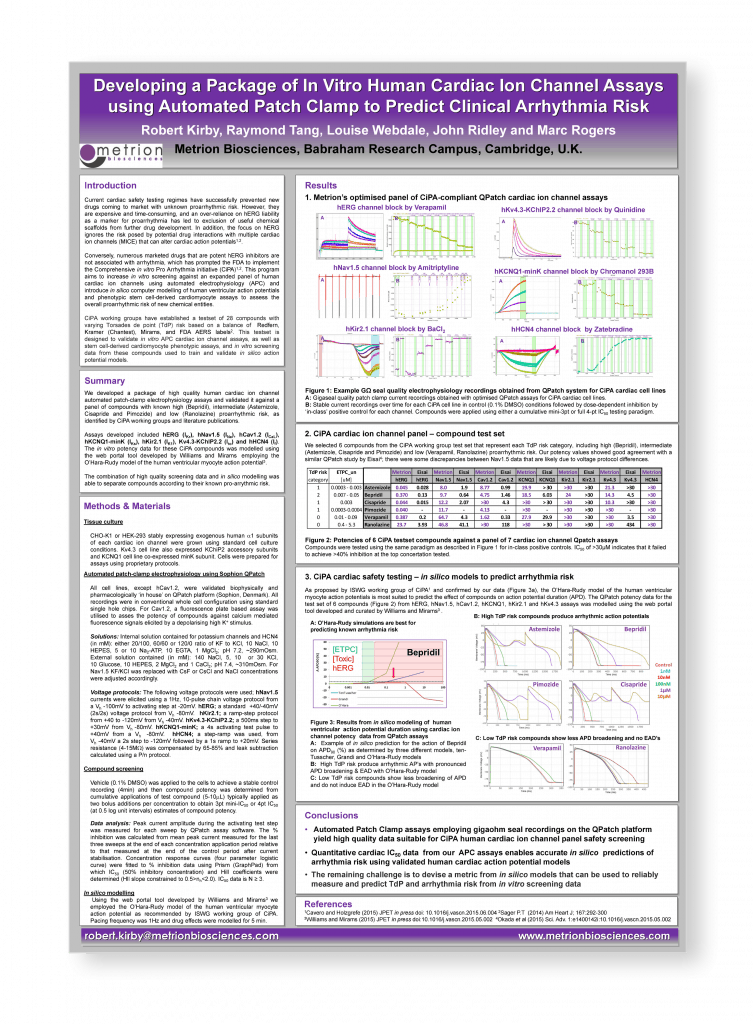
Developing a package of in vitro human cardiac ion channel assays using automated patch-clamp to predict clinical arrhythmia risk
Safety Pharmacology Society (SPS) Annual Meeting, Prague, 2015
Introduction
Current cardiac safety testing regimes have successfully prevented new drugs coming to market with unknown proarrhythmic risk. However, they are expensive and time-consuming, and an over-reliance on hERG liability as a marker for proarrhythmia has led to exclusion of useful chemical scaffolds from further drug development. In addition, the focus on hERG ignores the risk posed by potential drug interactions with multiple cardiac ion channels (MICE) that can alter cardiac action potentials.
Ion Channel Retreat, Vancouver, 2015
Introduction
The TTX-resistant sodium channel Nav1.8 is expressed in peripheral sensory nociceptors and is implicated in a range of inflammatory and visceral pain conditions such as irritable bowel syndrome (IBS)1. Nav1.8 channel function in sensory neurons changes after injury or inflammation, with a redistribution from the soma to axons and upregulated activity through inflammatory mediators and signalling pathways. This is thought to underlie increased neuronal excitability and a greater role of Nav1.8 currents in persistent, repetitive and ectopic action potential firing in inflammatory and visceral pain. Several gain-of-function mutations in Nav1.8 have also been detected in human patients suffering from small fibre neuropathy (SFN), offering similar genetic target validation and clinical population for personalised medicine approaches seen with Nav1.7 mutations in primary erythmelalgia, paroxysmal extreme pain and SFN patients.

Let’s work together
What are your specific ion channel and assay needs?
If you have any questions or would like to discuss your specific assay requirements, we will put you directly in touch with a member of our scientific team. Contact us today to discover more.