Written by Marc Rogers PhD and Sophie Rose PhD
A global pandemic wasn’t going to stand in our way

Nothing was going to prevent this annual gathering of ion channel enthusiasts and drug discovery professionals from taking place. Planning got underway in late 2020 to facilitate the virtual organisation and smooth running of the event. The event is usually held as a Satellite meeting at the Biophysical Society Annual conference, but could not be hosted virtually by BPS this year. Past sponsors Nanion, Sophion, Fluxion, SB Drug Discovery and Metrion Biosciences stepped in to prepare a schedule of ion channel experts from across the globe to present their recent work.
This year’s meeting brought together both academic and industry speakers from three different continents to talk about GABA(A) receptors and disease, potassium channel openers, neurotoxic venom peptides, with a keynote talk on viroporins that included recent data on drug repurposing against the SARS-CoV2 envelope (E) protein channel. To try and capture as wide an audience as possible, the meeting was held at 8am PST, 11am EST which captured audiences from both East and West Coast USA, the UK and Europe.
Hosting a virtual meeting with improved networking opportunities
Hopin was chosen as the meeting platform due to its enhanced networking capabilities compared to standard webinars we have all watched during lockdown. As well as an online text chat option for questions to be posed by attendees for the Speakers, visible online discussions could take place around each talk and session, and presentations were also interspersed with breakout sessions focusing on various areas of ion channel drug discovery which also promoted some enthusiastic discussions. A highlight for some of the attendees scattered over the globe was a video chat ‘roulette’, which allowed random pairings of attendees. The meeting was opened with a warm welcome from Marc Rogers (Metrion), followed by an introduction to the Hopin platform by Alexandra Stevens (Fluxion) and moderation of the event by Jason Villagomez (Nanion).
Keynote talk – An insight into viroporin virus ion channels as potential drug targets
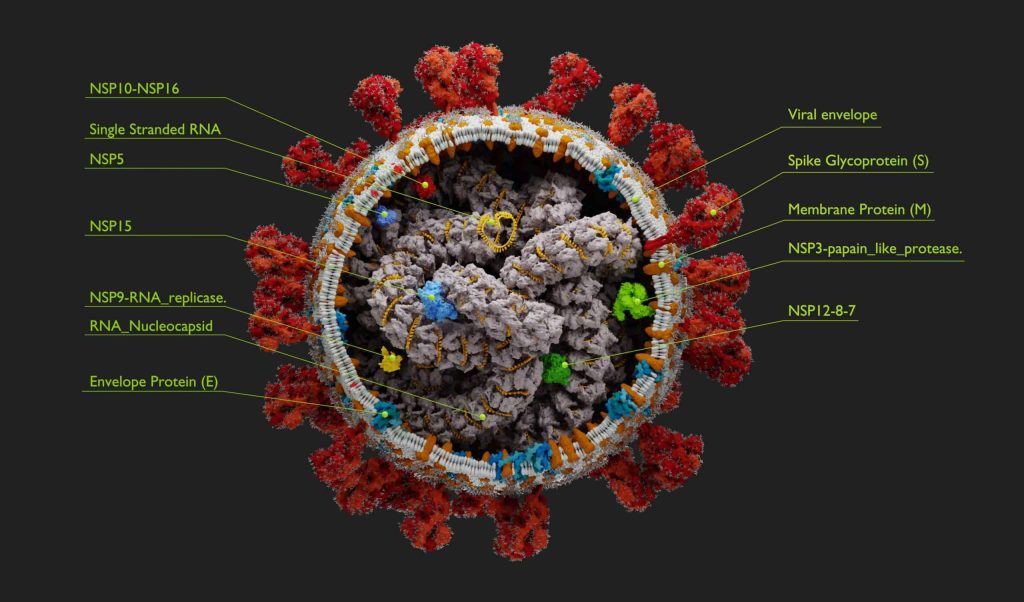
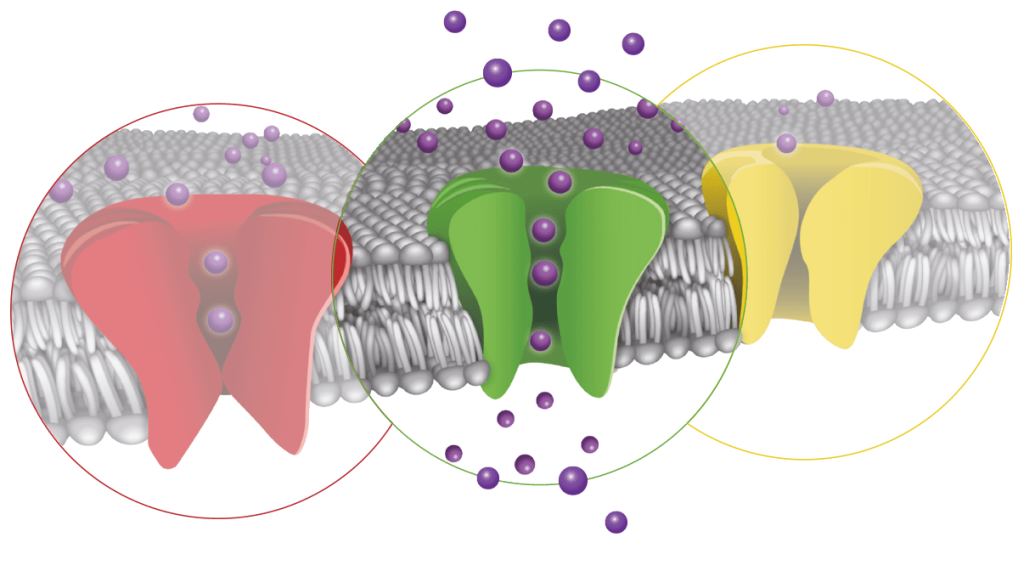
The Keynote talk was presented by Dr. Stephen Griffin (SG) from the University of Leeds who highlighted his wide-ranging work on viroporins, a group of small, multi-functional transmembrane proteins in disease-causing viruses which display ion channel like activity. SG introduced viewers to key viroporins including Hepatitis C p7, Zika M and influenza virus M2 proteins, and used both published work and unpublished data to illustrate how structure-function based rational development of inhibitors can be used to develop potent antivirals, including recent work to identify novel, drug-like modulators of the SARS Cov2 E3 envelope ion channel protein.
Development of antivirals has traditionally focused on disrupting DNA-RNA synthesis and replication (e.g. remdesivir), or as we are now all acutely aware, designing vaccines and antibodies to reduce virus infection (e.g. for HPV as well as SARS CoV2 coronavirus). Although viroporins are key to virus cell entry, vesicle trafficking and replication, they are under-exploited antiviral drug targets as little is definitively known about their structure. This is due largely to troublesome expression, and many of these putative ion channel proteins have multiple redundant functions, and reference ligands are promiscuous and non-selective, all of which can confound mutagenic studies and structure-activity relationship (SAR) screening. Several viroporins such as the influenza M2 and hepatitis p7 protein may first act as a pore rather than as channels, preventing the acidification of endosomal compartments that would prevent virus trafficking and release. However, there is mounting electrophysiological evidence that many viroporins exhibit the selectivity (permeation) and biophysical (gating) features of ion channels, and combinations of crystallographic data, NMR structures and molecular dynamic (MD) modelling are now revealing multiple binding sites and mechanisms amenable to further viroporin drug development.
The role of p7 viroporin in Hepatitis C function and drug development
SG started working on Hepatitis C in 2001 with a focus on p7 as a possible novel drug target as this viroporin acts during assembly, envelopment and secretion of viral particles. His group developed crystallographic and NMR structures and applied MD modelling to identify the binding sites of non-selective ligands such as rimantadine and amantadine, which were confirmed in functional liposome dye release assays. This binding site model was used in a virtual HTS to identify a diverse set of small molecules with improved potency and efficacy against viral particle release. SAR around the hit series has led to the development of the lead compound, JK3/32, which has similar potency to Sufosbuvir, a licenced antiviral to treat Hepatitis C. Significantly, this novel ligand pharmacophore is unaffected by mutations reducing rimantadine binding, raising the prospect of a new drug class less prone to developing drug resistance. SG also used their selective and potent lead compound to reveal a role for p7 viroporin in viral cell entry, suggesting that such small molecules could be used as prophylactics to prevent viral infection, which is especially important in patients unable to receive vaccinations.
Developing a synergistic approach to the treatment of Influenza M2
The next case study focused on the Matrix-2 (M2) protein, a viroporin located in the viral envelope of the Influenza A virus which causes seasonal ‘flu infections as well as the Spanish Flu pandemic 100 years ago. M2 was one of the first viroporins discovered and harbours mutations making it resistant to amantadine and rimantadine. Structure-based drug development against M2 was hampered by conflicting x-ray and NMR viroporin structures, but SG’s group sort to exploit this by looking for combination therapies that might mitigate evolution of resistance and reduce antiviral drug dosages. They generated two different families of M2 inhibitors, one targeting the viroporin lumen and the second targeting the peripheral channel binding site in the extended conductance domain, with selective binding modes and synergistic antiviral effects in cell culture against pandemic influenza virus strains. The novel compounds also showed favourable resistance profiles compared to adamantine derivatives.
Does the Zika Virus small membrane (M) protein form a Viroporin?
Zika virus is a recent mosquito-bourne disease vector which can cause neonatal microcephaly. The small envelope protein M forms a non-conducting dimer complex with the E envelope protein. However, this is known to dissociate in acidic endosomes to form a homomultimer which displays viroporin activity in liposomal assays, is pH activated and inhibited by rimantadine, as-is Zika virus cell entry and in vivo infection. Molecular modelling with the zika M protein viroporin structure has identified known and novel ligands that bind to luminal and peripheral pockets with nM potency and inhibit Zika virus replication in culture.
Drug repurposing targeting the SARS-CoV2 envelope (E) protein viroporin
Covid-19 is the most pronounced virological event in recent history and has had a significant impact on the entire world. As viroporins are varied with little homology, drug discovery efforts against each virus must rely on functional screening and reliable protein structures for molecular modelling, all of which kicked-off in early 2020 at the start of the coronavirus pandemic. SG explained that the SARS CoV2 virus may possess three different viroporins, but the most important pathogen is the E3 envelope protein. This has some similarities to related SAR and MERS pandemic coronaviruses envelope proteins in terms of function during viral entry, assembly and egress.
Before the release of verified E3 viroporin structures in mid-late 2020, suitable for MD modelling and virtual HTS, SG’s group used their liposome dye release assay to screen a library of FDA approved drugs for potential hits. Although amantadine and rimantadine were identified, their potencies against SARS-CoV2 (200 mM) are even lower than against other viroporins. In contrast, the repurposing screen identified a number of more efficacious inhibitors of viroporin activity at 400 nM, with diverse SAR. A recent membrane lipid structure of the E3 viroporin shows consistent virtual binding of many of the repurposed hits in a luminal pocket at the top of the tetrameric ion channel complex. This is in addition to showing activity in cell-based virological assays, paving the way for additional structure-based drug design and potential clinical development of small molecule antivirals for covid-19.
David Baez-Nieto – High-throughput automated patch clamp analysis of disease-related Cav3.3 channelopathies
The important role of Cav3.x channels
Dr Baez-Nieto, from the Stanley Center for Psychiatric Research at the Broad Institute in Boston presented a talk detailing the biophysical analysis of rare mutations in the neuronal CACNA1I gene that are associated with schizophrenia. So-called T-type or low voltage activated Cav3.x channels play important roles in sub-threshold oscillations and excitability in the peripheral and central nervous system, and through this function and disease-associated mutations they are viewed as validated drug discovery targets for pain and epilepsy.
Work from the Stanley Centre and others has now implicated the Cav3.3 subtype in schizophrenia, based on GWAS studies and patient genotyping that have revealed ~60 ultra-rare missense variants. The challenge is to determine what effect each mutation may have on Cav3.3 channel function, and how this may relate to disease risk, occurrence and severity. Each rare mutation was expressed heterologously and the Syncropatch384 automated patch clamp (APC) platform was used to determine the subtle biophysical differences between each Cav3.3 mutant, such as voltage-dependence and recovery from inactivation. Meaningful differences were found based on a large number of recordings made from many cells in parallel to allow for sophisticated data analysis and statistical comparison.
Significantly, common schizophrenia variants were well tolerated and generally did not affect Cav3.3 biophysics or expression. Unexpectedly, several of the ‘unaffected’ patient mutations reduced Cav3.3 current expression but had little effect on channel biophysics, suggesting that function was maintained by those channels reaching the plasma membrane of thalamic neurons in vivo. Finally, there were a range of subtle differences in expression as well as activation and inactivation biophysics for many of the rare schizophrenia-associated mutations. These differences were visualised with ‘spider web’ or radar plots, which revealed no significant changes in recovery from inactivation for any mutant group.
Studying schizophrenia mutant Cav3.3 biophysics
In collaboration with Diane Lipscombe at Brown University, schizophrenia mutant Cav3.3 biophysics were added to a computational model of thalamic neurons to predict functional effects on firing. As expected, common mutations did not affect thalamic firing, but altered Cav3.3 activation seen in rare schizophrenia mutants changed action potential burst firing latency, threshold and spike output. This revealed groups of unaffected and rare disease mutations that were either gain or loss-of-function in terms of thalamic firing; the latter mutations may be protective in relation to schizophrenia symptoms.
Such functional and integrative multi-parameter data is critical for the correct annotation of rare channelopathies, and improved pharmacogenomic treatment of individual patients. This talk reveals the utility of high throughput gigaseal APC platforms as ‘biophysical machines’ for disease-related channelopathy investigations, alongside their proven role as ‘compound screening’ machines for ion channel drug discovery and safety pharmacology profiling.
John Atack – GABAA Receptor Modulators, The Story Continues
GABA-A receptors and modulators
Prof. Atack is Director of the Medicines Discovery Institute at Cardiff University in the UK and has worked in the field of ligand-gated GABA-A receptors for many years. He started his talk with an overview of the structure-function of GABA-A receptors, noting the various key heteromeric isoforms implicated in various disease states and human physiology such as anxiety, epilepsy, anaesthesia, and their modulatory ligands such as barbiturate activators and benzodiazepine (BZP) and neurosteroid positive allosteric modulators (PAM). He highlighted several recent FDA approvals for GABA-A modulators, notably the neurosteroid allopregnanolone PAM Brexanolone for post-partum depression (Sage Therapeutics), and the BZP sedative anaesthetic Remimazolam (Acacia). Although BZPs can be very effective anxiolytics and muscle relaxants, chronic use for long-term disease is problematic owing to their sedatory side-effects and development of tolerance and addiction.
Medium and high throughput screening of small molecules
Development of non-sedating BZPs has been facilitated by recent cryoEM structures of mammalian GABA-A receptors comprising the relevant a (1, 2, 3, 5), b3 and g2 subunits. Thus, new BZPs with reduced sedation should be designed to be less active at a1-containing receptor heteromers that are widely distributed across the CNS, whilst anxiolytic and cognition-enhancing efficacy of BZP ligands can be achieved through selective PAM of a2/a3 and a5 subunit-containing heteromers, respectively. Interestingly, whilst some groups have attempted to create scaffolds with selective binding affinity for certain GABA-A receptor subtypes, more success was seen using a selective efficacy approach such that binding to undesired subunit stoichiometries was ‘silent’. The latter methodology forms the basis of the work in Cardiff, which started screening small molecules in-house on the medium-throughput QPatch-16 automated patch clamp platform, and has recently expanded to use the Syncropatch 384 HTS robot in collaboration with Scottish Biomedical. Both assay platforms were validated using preclinical and clinical candidates (e.g. AZD7325 and PF-06372865) and showed very similar isoform selectivity and potentiation efficacy profiles, which also aligned with literature ‘gold standard’ manual patch data.
Test compounds from Prof Atack’s medchem group were normalised to those of diazepam, revealing a lead compound MDI-595 with ~50% of the efficacy of diazepam at a2/a3 but only 20% activity against a5 and no activity against sedatory a1 GABA-A receptors even though it bound with pM affinity. This compound is brain penetrant and achieves good GABA-A receptor occupancy with an ED50 of ~40ng/ml plasma exposure, and produces significant anxiolytic activity in the elevated maze with an MED < 1 mpk after oral dosing without any side-effects in a rotarod assay up to 30 mpk. This promising profile should support the preclinical IND profiling of a lead series candidate in 2022 followed by clinical testing in 2023.
Julie Klint – Drug discovery of Kv7.2/7.3 openers
Our next speaker was Dr. Julie Klint, who is Head of the Ion Channel section (Molecular Screening) at Lundbeck in Denmark. She and her colleagues had just published a paper describing the successful preclinical development of a selective M channel (Kv7.2/7.3) modulator for neuropsychiatric disease, and here she went into detail on the complex multi-parameter voltage protocols employed at Lundbeck (oocytes) and Metrion Biosciences (CHO cell lines) to profile the potency, selectivity and mechanism-of-action of their lead series compounds, and how they compared to the reference clinical drug, Retigabine. This family of ion channels are widely expressed in cardiac tissue (KCNQ1) and neurons (KCNQ2 – 5), where their activation at resting membrane potentials can strongly modulate single and repetitive action potential firing. Thus, Kv7.1 modulators carry cardiac risk, whilst neuronal Kv7x openers may have utility to reduce firing in pathophysiological conditions and diseases such as pain, epilepsy, ALS and depression.
Screening for Kv7 modulators
Due to the complex biophysics of Kv7 channels and the difficulty in aligning a particular voltage protocol readout or experimental parameter to functional effects on neuronal firing, many groups have used a variety of end points to assay for Kv7 modulators. These include measurements of maximal current or activation, shifts in the voltage-dependence of half-activation, or alterations in current deactivation kinetics. It is unlikely that just one parameter is sufficient, and using a single readout for SAR screening runs the risk of missing useful chemical scaffolds. Thus, more recent efforts have tried to use complex protocols, as exemplified by Lundbeck’s testing in Xenopus oocytes where they included an action potential-like waveform alongside measures of sub-threshold and maximal activation, as well as the voltage-dependence and rate of current activation. Similarly, Metrion developed a complex voltage protocol for screening test compounds on the QPatch platform which is able to distinguish between several different reference compounds with different Kv7 channel binding sites and modulatory mechanisms. Thankfully, the modulatory profile of the Lundbeck compounds aligns well between the client and CRO sites and their respective complex voltage protocols.
The in vitro patch clamp efficacy of the Lundbeck lead compound Lu-AA411178 matches that of the clinical reference Retigabine, and mutagenesis studies suggest that Lu-AA41178 binds to the same or similar site to Retigabine on neuronal Kv7 channels. The lead compound demonstrates in vivo efficacy at low doses (but a narrow therapeutic index) in a panel of relevant CNS disease assays such as MES and PTZ seizure, depression and anti-psychotic models, thanks to brain penetrant PK. Significantly, the lead compound is relatively selective within the KCNQ family (including cardiac Kv7.1), has no other cardiac ion channel liability, and is clean in a wide profiling screen including the lack of any GABA-A activity seen with Retigabine. It is believed that in vivo CNS disease efficacy of the promising Lundbeck compound is likely driven by subtle changes in activation, but a more drug-like molecule will be needed for clinical development.
Julie’s talk elicited some spirited discussions around the ability of a HTS patch clamp platform to deliver such high content data at an early stage of screening (shout-outs to Sarah Lilley at Sussex University, Damian Bell at Sophion and Tim Strassmeier at Nanion), a level of diagnostic detail that is frequently missing from traditional plate-based screening assays.
Irina Vetter – Neurotoxic venom peptides from the giant Australian stinging tree
As if Australia didn’t have enough dangerous animals ready to eat, bite and sting people, Prof. Irina Vetter’s group at the Institute for Molecular Bioscience & School of Pharmacy at the University of Queensland have described another venomous assailant, namely stinging trees. What is particularly fascinating about this story is that it could be another example of convergent toxin evolution, as the plant peptides bear a striking structural resemblance to a class of spider toxins known to modulate Nav channels.
The gympie gympie tree and a new family of ‘gympietides’
Gympie gympie trees are related to European stinging nettles but grow to a much larger size as bushes or trees, and their leaves and stems are covered in stinging hairs (silica-rich trichomes) that can inject venom which elicit profound and long-lasting pain. Irina’s group used activity-guided HPLC fractionation to identify a single 4 kDa fraction that elicited nocifensive reactions in mice, and subsequently identified a new family of disulphide-rich ‘gympietides’ and venom gene sequences. Although these peptides were dissimilar in sequence to known plant or animal-derived toxins, their triple Cys-loop 2D and 3D structures were analogous to inhibitory cysteine knot (ICK) toxin peptides found in spiders and cone snails. Functional testing of synthetic gympietides confirmed their predicted action to modulate the activity of mammalian TTX-sensitive Nav channels in rodent DRG neurons via impaired current inactivation.
Gympietides as potential tool compounds
Biophysical experiments using mammalian cell lines on the QPatch revealed opposite shifts in the voltage-dependence of activation and inactivation to produce persistent window currents close to DRG neuron resting potentials, with more potent activity seen for the more painful stinging tree peptide. Gympietides activate Ca2+ signalling in isolated DRG neurons, increase peripheral sensory nerve firing in an ex vivo rodent model, and produce profound pain behaviours when injected into the footpad of mice which are reversed by TTX and a selective Nav1.7 inhibitory toxin Pn3a. Thus, although gympietides may not be particularly selective for Nav1.7 (question from Sam Goodchild at Xenon and Tianbo Li from Genentech), their novel sequences and potential as a tool compound could be useful for Nav-specific target engagement in pain assays and other drug discovery applications.
Andrew Jenkins – GABA(A) receptors and disease
Prof. Jenkins is in the Dept. of Anesthesiology, Pharmacology & Chemical Biology at Emory University School of Medicine in the USA. As we heard in Prof. Atack’s talk, there is a great deal of interest and research around GABA-A PAMs such as benzodiazepines, neurosteroids and anaesthetics, and their potential utility in certain neurological diseases vs their sedatory side-effects which affect the balance between excitatory and inhibitory signalling in brain regions that regulate arousal and sleep.
Prof. Jenkins is interested in the potential role of GABA-A receptors and synaptic signalling in patients with idiopathic hypersomnia, who suffer from excessive daytime sleepiness and poor sleep patterns but lack a definitive clinical, genetic or biological cause of their ailment. His earlier work had identified a small peptide fraction in patient CSF that potentiated GABA-A currents and was sensitive to a benzodiazepine binding site antagonist, whilst a smaller effect was seen in control patient CSF samples, suggesting that there is a normal sleep-modulating peptide in human brain that may be dysregulated in hypersomnia patients.
Screening patient samples
As CSF sampling and testing against human GABA-A receptors may provide a definitive diagnosis for such patients, and have potential for pharmacological profiling and patient stratification, it became clear that a higher throughput and more reproducible electrophysiology screening system was needed to move the project beyond manual patch clamp. Joe Lynch’s group in Australia were obtaining similar results with the medium throughput Patchliner platform that correlated very well with the Emory manual patch data, but throughput was still insufficient to test multiple replicate samples from a growing list of hypersomnia patients. Accordingly, Prof. Jenkins turned to the microfluidic Ionflux APC platform, which offers both higher throughput and reduced sample volumes, helping scale-up patient screening and deliver reproducible data across multiple and sometimes highly variable CSF samples. As well as patient CSF profiling and diagnosis, Prof Jenkins and his collaborators began work to identify the specific sleep-modulating peptide(s) in patient samples using the IonFlux Mercury 384 platform. Of 62 up-regulated proteins identified from patient vs ‘ultra clean’ control CSF proteomes, several have been confirmed to high statistical significance, including a wildtype peptide (which may be up-regulated in patients vs controls) as well as several patient-derived peptide variants. He also used the same Ionflux screening assay to identify potential negative allosteric modulators of sleep-inducing GABA-A receptors to treat hypersomnia patients through a drug repurposing approach, which has identified hydroxychloroquine and clarithromycin (both hits against covid-19 infection as well). Finally, a metabolomics analysis of CSF samples from 44 patients identified over 200 GABA-A potentiators and over 300 negative modulators, which are now being confirmed by electrophysiology. Whilst it had taken 10 years to screen 600 patient samples by manual patch clamp, utilisation of a microfluidic APC platform now allowed a 10-300 fold reduction in sample volume use and screening time (e.g. 600 patient samples in 12 weeks), greatly facilitating patient diagnosis and insights into the underlying disease mechanisms.
Prof. Jenkins finished his talk by describing recent work showing the preferential expression of functional a5 containing GABA-A receptors in cancer cell lines, and evidence that a5-preferring benzodiazepine PAMs can accelerate cancer cell death. Thus, GABA-A PAMs offer the potential to be used alongside conventional chemo- and radio-therapy treatments to increase efficacy and reduce the well-known side-effects of cancer drugs.